

- Home
- Companies
- Alliance Spine
- Products
- Alamo - Model T - Transforaminal Lumbar ...
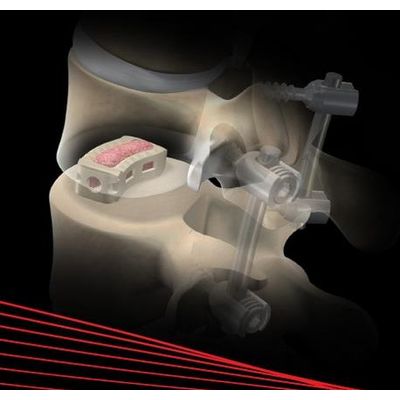
Alamo - Model T - Transforaminal Lumbar Intervertebral Device
Transforaminal lumbar intervertebral device manufactured from PEEK Optima LT1 with tantalum markers for radiographic visualization.
- Length x Width x Height lordotic configurations available:
- 32 x 11 x 8 through 14mm (in 1mm increments)
- 28 x 11 x 6 through 14mm (in 1mm increments)
- 0° and 7° Lordosis
- Large Grafting Area
- Tapered Nose For Ease of Insertion
- Aggressive Teeth for Secure Fixation
- Tantalum Markers for Implant Visualization
- 0° Lordosis:
The Alamo®T is intended for spinal fusion procedures in skeletally mature patients with degenerative disc disease (DDD) at one or two contiguous levels of the lumbosacral spine (L2-S1).
DDD is defined as back pain of discogenic origin with the degeneration of the disc confirmed by history and radiographic studies. These patients should have had six months of non-operative treatment prior to treatment with an intervertebral cage.
In addition, these patients may have up to Grade 1 spondylolisthesis or retrolisthesis at the involved levels.
The device system must be used with supplemental fixation and autograft to facilitate fusion and is to be implanted via a transforaminal approach.
