


PUMA-G - Ultrasound Gastrostomy System
No endoscope; no ionizing radiation. The PUMA-G System eliminates risks from PEG blind sticks (organ damage); endoscope reprocessing (infections); or fluoroscopy (cancer) by enabling ultrasound visualization of tissue from stomach wall to surface.
The FDA-cleared PUMA-G System has been validated with over 250 safe and successful uses in adult patients (including COVID+ patients), confirming prior performance aspects shown in live canine and cadaver data that demonstrate the method`s effectiveness and reliability.
Compared to the total cost of PEG procedures, the PUMA-G System delivers a >30-50% reduction in direct costs, major savings from shorter lengths of stay, and revenue enhancement through optimization of suite productivity.
Scheduling logjams for an operating suite can delay patient care and increase costs. Gastrostomy procedures done at the bedside leverage the skills of physicians at the point of care and results in more efficient patient care.
A study was composed during the fourth quarter of 2020, to explore the additive safety measure that the use of the PUG system allows health providers. To access the study abstract, click the link below.
A new 25 patient study out of Columbia University compares PUG with PRG retrospective analysis. Results demonstrate that PUG (1) is safe bedside procedure; (2) can be performed by ultrasound trained physicians; and (3) reduces transport and care delays in COVID-19.
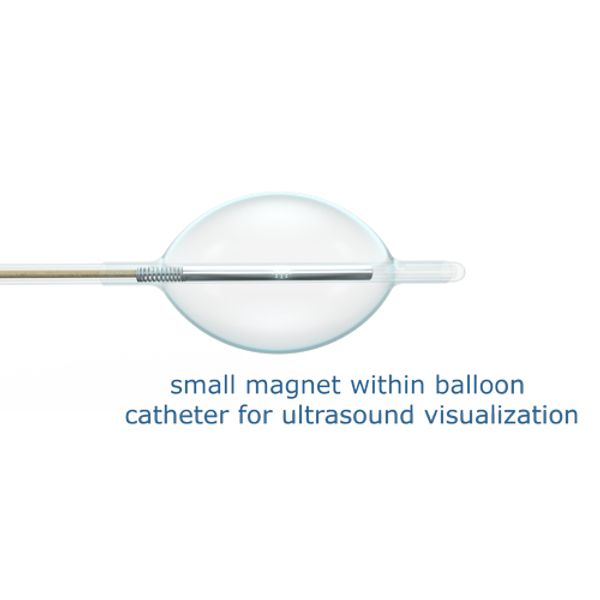
- French Size: 16
- Sterilization: EO
- Length: 40" / 101.6cm
- Shelf Life: 3 years
Designed for use with two hands, enabling one physician to work in the sterile field.
