

Matica Biotechnology, Inc.
- Home
- Companies
- Matica Biotechnology, Inc.
- Services
- CDMO Services
CDMO Services
Our viral vector and cell therapy manufacturing platforms have been designed to streamline the CMC development cycle by optimizing biological components, processes and documentation to maximize titers, minimize impurities, and mitigate technical and regulatory risks.
Most popular related searches
cell line development
infection assay
viral vector
cell therapy
immunoassay
infection test
sterility testing
immunoassay test
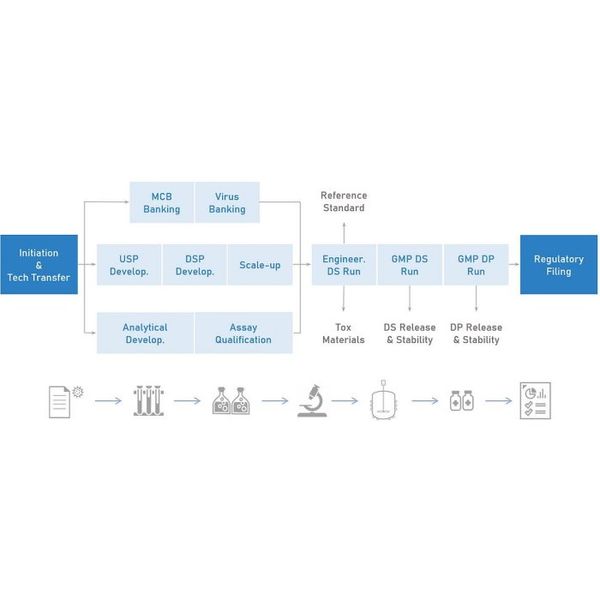
1. Cell Line Development
- Clonal selection and growth parameters
- Adaption to suspension-based growth
- Media screening and cell culture optimization
- Cell banking
2. Process Development
- Vector rescue & development
- Plasmid transfection efficiencies
- Bioreactor parameter optimization
- Improved product profiles
- Streamlined scale-up & transfer to GMP production
3. Assay Development
- Method transfer, development & optimization
- Phase-appropriate validation & transfer to QC testing lab
- Development of GMP grade bill of materials and raw materials testing program
4. GMP Production
- Full utilization of single-use technologies
- Suspension or adherent processes
- Production up to 500L scale
- Purification utilizing large-scale filtration and chromatographic unit operations
5. Product Release and Stability Testing
- Viral particle titers
- Infectivity assays
- Molecular assays
- Immunoassays
- Sterility analyses
- ICH stability chambers
