Research and Development Services
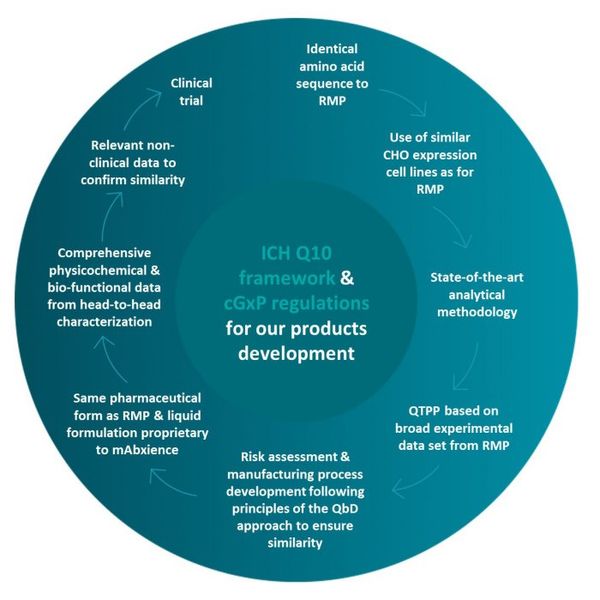
In-house, core R&D competencies provide differentiated development capabilities for complex biopharmaceuticals. End-to-end expertise and capabilities across development of the cell line, formulation, upstream and downstream processes, and fill & finish. R&D platform ensures maximum control over quality, efficacy and development of biopharmaceutical medicines adhering to a strict “quality by design” standard. Leveraging cutting edge analytics of the latest innovations in downstream & upstream process development. mAbxience network centers of excellence is in an ongoing expansion with various locations in the European Union and Latin America, and will be consolidated with our brand new 940sqm state-of-the-art biopharma R&D center in León (Spain) which is expected to open in 2022.
- CHO: Chinese Hamster Ovary
- QTPP: Quality Target Product Profile
- RMP: Reference Medicinal Product
- QbD: Quality by Design
- ICH: International Conference on Harmonization
- cGxP: Current Good X Practice (X can mean: Clinical, Laboratory, Manufacturing, Pharmaceutical)