

- Home
- Companies
- qmsWrapper
- Software
- qmsWrapper eQMS - Integration Software ...
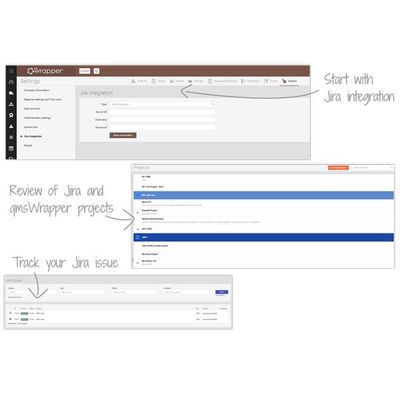
qmsWrapper eQMS - Integration Software with Jira
The combination of Jira with qmsWrapper introduces a system in which MedDev companies are able to track, manage, and organize all CE Technical Files and FDA Design History Files, while enabling software development of medical devices.
Jira platform help teams plan, assign, track, report and manage development projects. But you need much more to stay compliant and to get your device to market!
With qmsWrapper, Jira users can accomplish everything from one place. They will have a complete overview in qmsWrapper of all tasks issued in Jira, and all available functionalities that support your way to compliance! Put differently, Jira is “wrapped with QMS”.
All projects created in Jira are visible in qmsWrapper’s Project Management Module, hence is easy for you to follow up. Tasks can be organized in the Traceability Matrix which ensures traceability to Design Controls.
This integration will help all medical device companies reduce development risks and increase agile effectiveness, whilst still keeping an eye on quality!
qmsWrapper provides all the essential functionalities for the implementation of your QMS. You can record all quality events like non-conformities, CAPAs or training since this unique software is made to support compliance.
Staying compliant is the #1 priority for all medical device companies— but it doesn’t have to be difficult. Jira platform represents a choice for intuitive, streamlined solutions for ongoing Project Management and collaboration, but through qmsWrapper is just the perfect environment for easy Quality Management.
