

Neuspera Medical, Inc.
- Home
- Companies
- Neuspera Medical, Inc.
- Software
- Neuspera - iPad-based Clinician ...
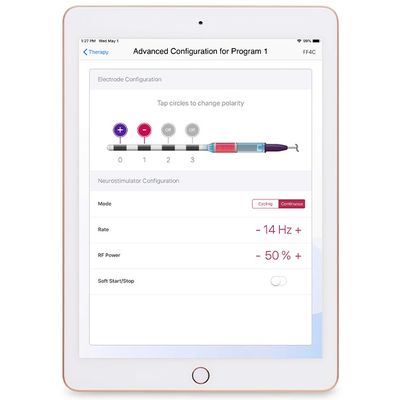
Neuspera - iPad-based Clinician Programmer
An iPad-based clinician programmer will give physicians a “command center” view of all Neuspera implanted devices and external wireless transmitters within their practices. The programmer’s advanced functionality allows clinicians to modify a number of treatment parameters for patients, change electrode configurations, set stimulation program specifications, and pair one or more wireless transmitters to an implant. Data visualization features display usage and other useful information to help physicians work with patients to optimize therapy.
