Vaccine Manufacturers Articles & Analysis
13 articles found
This article delves into the pivotal functions of enzymes in mRNA vaccine manufacturing, highlighting their significance in ensuring vaccine efficacy and safety. ...
By understanding the multifaceted applications of glycerin in vaccine formulation, storage, and delivery, pharmaceutical manufacturers can optimize their vaccine production processes to ensure superior quality and effectiveness. ...
This article explores the intricate and indispensable role that raw enzymes play in the manufacturing of mRNA vaccines, delving deep into their significance and impact on vaccine production. As we venture into this fascinating world, we will gain a comprehensive understanding of how these enzymes are the unsung heroes behind the remarkable ...
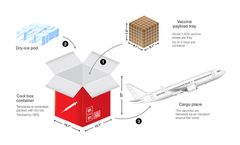
Due to the particularity of vaccines, vaccines need to be kept in a constant and suitable temperature environment during transportation and storage, and different vaccines require different environmental temperatures. ...
From the point of manufacturing until the last mile, the vaccine cold chain includes a series of interconnected steps: Manufacturing and quality control Vaccine manufacturers adhere to stringent quality control measures to ensure the vaccines’ potency and safety. ...
Avian cell lines for human and veterinary vaccine manufacturing Currently, many NDV-based vaccines and viral vectors are still produced using a traditional process in embryonated chicken eggs or primary chicken embryo fibroblasts (CEF). ...
ByNuvonis
One of the major challenges is the manufacturing of a new SARS-CoV-2 vaccine in large scale for global demand. So far, only few companies use a conservative but tested approach of producing a classical inactivated vaccine, for which production capacity can be easily found. Several years ago this concept was demonstrated successfully by Baxter ...
ByNuvonis
Our ultra-low temperature freezers are used for the storage of drugs, vaccines, enzymes, chemicals, bacteria and other samples. Vaccine Storage: Cold Chain Vaccine storage requires that vaccines are stored within a set temperature range from the point of manufacture to vaccination. ...
Influenza vaccine manufacturing is an intricate and complex process that culminates in the summer months, when U.S. flu vaccine manufacturers receive regulatory approval from the FDA for the season’s vaccines. The manufacturers then prepare to distribute millions of doses to health care ...
It requires the cooperation between influenza vaccine manufacturers like Seqirus, and the World Health Organization’s (WHO) 158 laboratories in 127 countries and 7 collaborating centers that comprise its Global Influenza Surveillance and Response System, or GISRS. ...
As of October 2021, 6-7 billion doses of COVID-19 vaccines have been provided under emergency measures. The vaccines roll out has peaked at 45 million doses/day and has remained broadly stable (Our Word in Data & WHO), so we can assume that this is the current global manufacturing capacity. ...
The vaccine cold chain begins with a cold storage unit at the vaccine manufacturing plant, extends to the transport and delivery of the vaccine (including proper storage at the provider facility), and ends with the administration of the vaccine to the patient. A breakdown in protocols anywhere along the cold ...
Unlike medicines, which are used to treat or cure diseases, vaccines are intended to prevent them. Handling and Storage of Vaccines Developing a vaccine can take years before it is deemed safe for human use and, thereafter manufactured and made available for widespread distribution and inoculation. Throughout the ...