Vaccine Producing Articles & Analysis
41 articles found
Once synthesized, these proteins spontaneously assemble into VLPs, providing a scalable and efficient way to produce vaccines. Applications in Vaccination VLPs have shown considerable promise in the field of vaccination. ...
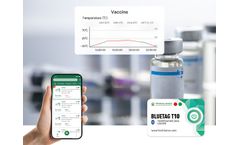
The optimal storage environment temperature for most vaccines is 2-8℃. It is necessary not only to control the temperature stability but also to monitor the temperature. The 2-8℃ Vaccine Bluetooth Temperature Sensor can help managers view the storage environment temperature data directly on the APP without opening the incubator, thereby improving the efficiency of cold chain temperature ...
By utilizing recombinant DNA technology, scientists can produce vaccines that are more effective and safer for public use. Understanding Recombinant Vaccines Recombinant vaccines are created by inserting genetic material from a pathogen into a host cell. ...
Recombinant protein vaccines are vaccines produced using genetic engineering technology. The principle is to clone one or several antigen genes of a pathogen into host cells, causing them to express the corresponding antigen protein. These proteins are then extracted for use in the vaccine. These vaccines can ...
The advent of mRNA vaccines has revolutionized the field of immunization, offering a rapid and adaptable response to emerging infectious diseases. Central to the production of these vaccines are enzymes, which play crucial roles in the synthesis and modification of mRNA molecules. This article delves into the pivotal functions of enzymes in mRNA vaccine manufacturing, highlighting their ...
Recombinant protein vaccine is a type of vaccine that does not contain complete pathogens and is prepared from specific protein antigens produced by heterologous expression systems. ...
Custom mRNA production has emerged as a cutting-edge technology in the field of molecular biology, offering researchers the ability to tailor-make specific messenger RNA molecules for various applications such as vaccine development, gene therapy, and regenerative medicine. This innovative process holds great promise in unlocking new possibilities in scientific research, paving the way for ...
Introduction RNA Lipid Nanoparticles (LNP) technology has rapidly gained prominence in the health and scientific domain, primarily due to its significant role in carrying the mRNA of the COVID-19 vaccines developed by Pfizer/BioNTech and Moderna. Yet, the potential of this technology spans beyond the confines of these vaccines or even the health industry. ...
However, despite the fact that aluminum adjuvants in vaccines have been used in enhancing vaccine immune responses for nearly decades, the molecular mechanisms of aluminum adjuvants are still not fully understood. ...
By understanding the multifaceted applications of glycerin in vaccine formulation, storage, and delivery, pharmaceutical manufacturers can optimize their vaccine production processes to ensure superior quality and effectiveness. ...
Vaccines are considered one of the most successful medical interventions in the past few centuries, aiming to harness the human immune system and generate lasting protection against specific diseases. Traditional vaccines rely on the use of inactivated pathogens to trigger an immune response. However, many of these formulations carry a high risk of causing allergies or autoimmune reactions. ...
This article explores the intricate and indispensable role that raw enzymes play in the manufacturing of mRNA vaccines, delving deep into their significance and impact on vaccine production. ...
Due to the particularity of vaccines, vaccines need to be kept in a constant and suitable temperature environment during transportation and storage, and different vaccines require different environmental temperatures. ...
Host Cell Residual DNA (rcDNA) refers to fragments of DNA derived from host cells that may be present in biological products. These products must not contain foreign substances, particularly host DNA, to avoid immune rejection and potential threats to life safety. Regulatory agencies worldwide have imposed strict limits on the amount of rcDNA, and various pharmacopoeias have outlined several ...
This finding will shed more light on the range of CPS types to include in future vaccines, as current vaccines against S. pneumoniae do not include many types produced by the bacteria CPS," added Jade Chun from the Department of Microbiology and Immunology, NUS Faculty of Medicine. About the author Collected by CD BioGlyco, a biotechnology ...
Avian cell lines for human and veterinary vaccine manufacturing Currently, many NDV-based vaccines and viral vectors are still produced using a traditional process in embryonated chicken eggs or primary chicken embryo fibroblasts (CEF). ...
ByNuvonis
The pandemic caused by the virus has unforeseen effects on the global economy and modern society, leading to a global race to find a suitable treatment and a preventive vaccine. SARS-CoV2 vaccine development A large number of companies operating in the vaccine field announced that they are working on a new COVID-19 ...
ByNuvonis
Vero cellsare a continuous adherent cell line that is frequently used in cell culture and plays an important role in worldwide vaccine and vector production (manufacturing). Unlike other mammalian cells, they do not secrete interferon alpha or beta when infected by viruses – this is one of the reasons that efficient replication of many viruses can be ...
ByNuvonis
The World Health Organization in its report on Neglected Tropical Diseases has stated that there is overwhelming evidence to show that the burden caused by many of the 17 diseases that affect more than 1 billion people worldwide can be effectively controlled and, in many cases, eliminated or even eradicated. Leishmaniasis caused by Leishmania spp is one such example and poses a grave health risk ...
Abera’s vaccine delivery platform works as a plug-and-play system where known or novel antigens can be engineered onto our delivery platform to create effective, multivalent vaccines that are cost-effective and fast to produce. We actively work together with academia and industry to enable the use of our vaccine delivery ...