Altimmune Inc. products
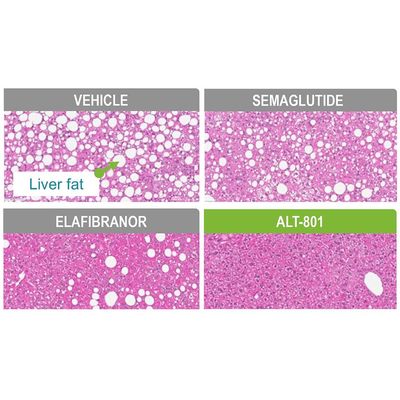
Dual GLP-1/Glucagon Agonist For NASH
A peptide-based dual GLP-1/glucagon receptor agonist designed to treat the metabolic dysfunction that causes non-alcoholic steatohepatitis (NASH). NASH, the most severe form of non-alcoholic fatty liver disease (NAFLD), involves multiple metabolic pathways leading to the abnormal accumulation of liver fat, toxic lipid metabolites, and inflammation, leading to fibrosis or eventually liver cancer. Because ALT-801 acts across these multiple pathways, it may be able to correct the metabolic dysfunction at the heart of NASH as demonstrated in one of the best preclinical models of the disease.
HepTcell - Immunotherapeutic for Chronic Hepatitis B Infections
An immunotherapeutic product candidate for patients chronically infected with the hepatitis B virus (HBV). It is designed to drive CD4+ and CD8+ T-cell responses against all HBV genotypes in patients of diverse genetic backgrounds. Normally, the HBV virus is eliminated through T cell dependent mechanisms but stimulating these responses in chronically infected HBV patients has been challenging due to the exhaustion of T cells directed against the virus. HepTcell has shown the potential to break the immunotolerance characteristic of this disease and activate T cells to fight the infection.
NasoVAX - Intranasal Flu Vaccine With Diverse Immune Response
A recombinant intranasal vaccine that is being developed for both seasonal and pandemic use. NasoVAX can activate the humoral, mucosal and cellular immune arms in unison for a more comprehensive immune response. The data from our Phase 2a trial with a monovalent NasoVAX vaccine indicated that NasoVAX was well-tolerated and achieved 100% seroprotection with serum antibody responses comparable to a licensed injected influenza vaccine.
T-COVID - Single-Dose Intranasal Therapeutic for the Treatment of Early Covid-19
Identical vector technology used for AdCOVID (COVID-19), NasoVAX (seasonal influenza) and NasoShield (anthrax) vaccines.
NasoShield - Single Dose Intranasal Anthrax Vaccine
An intranasal vaccine designed to provide rapid, stable protection against anthrax after a single administration. It is being developed with the support of the U.S. Biomedical Advanced Research and Development Authority, (“BARDA”) for post-exposure prophylaxis against anthrax following exposure to aerosolized B. anthracis spores.