

Altimmune Inc.
- Home
- Companies
- Altimmune Inc.
- Products
- T-COVID - Single-Dose Intranasal ...
T-COVID - Single-Dose Intranasal Therapeutic for the Treatment of Early Covid-19
FromAltimmune Inc.
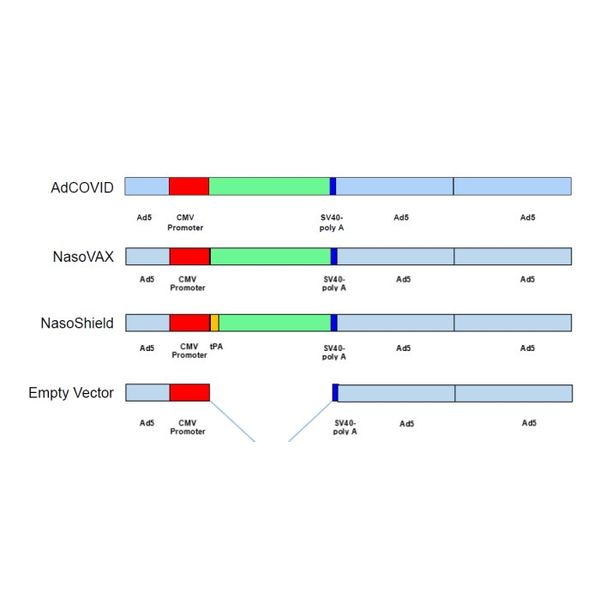
Identical vector technology used for AdCOVID (COVID-19), NasoVAX (seasonal influenza) and NasoShield (anthrax) vaccines.
Most popular related searches
COVID 19
clinical trial design
preclinical trial
innate immunity
influenza vaccination
influenza vaccine
mechanical ventilation
infection protection
influenza infected
influenza infection
Data from 6 preclinical studies of influenza infection funded by NIAID and conducted at Utah State University showed:
- Rapid, non-antigen mediatedmodification of host cytokine response
- Protection from lethal challengeoccurs within days and lasts for weeks
- Significantly decreased inflammationfollowing respiratory virus infection
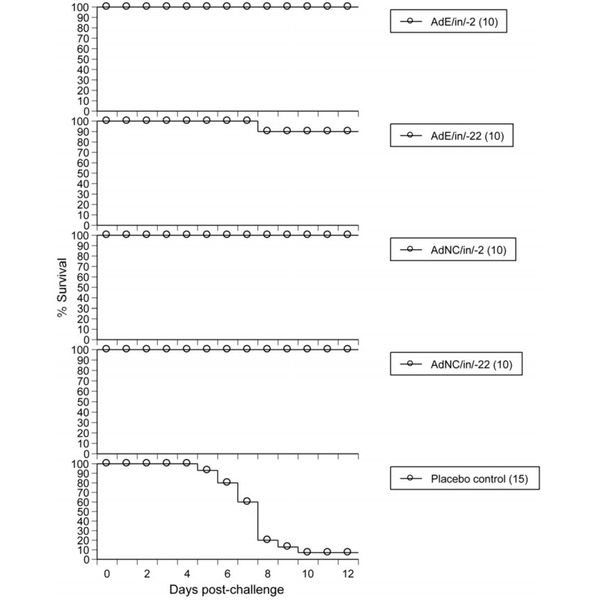
Experimental design
Day -2 or Day -22
- Intranasal administration (2.5 x108ifu) of either empty vector (vector without antigen) or NasoVAX (vector with antigen)
Day 0
- Challenge with influenza A/CA/04/2009 (3 x LD50)
Results
- Protection provided by both empty vector and NasoVAX
- Protection occurred when treated between 2-and 22-days prior to challenge
- Identical results obtained following challenge with other influenza A strains, influenza B, H5N1 and H7N9
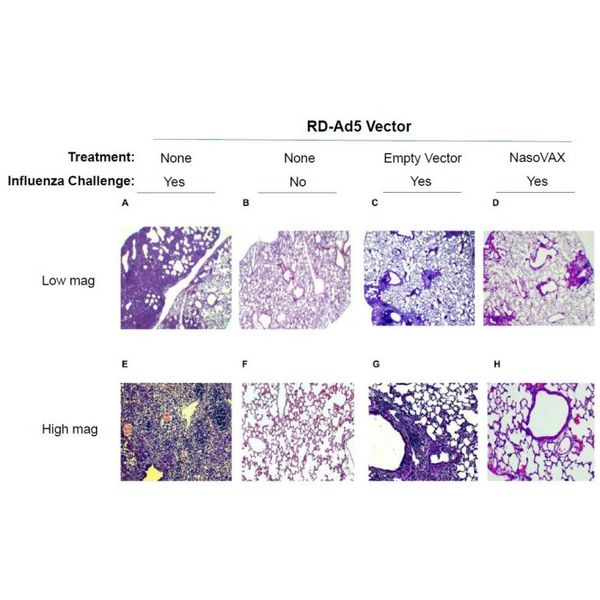
- Intranasal administration of either empty vector or NasoVAX on Day -2
- Challenge with influenza A/PR/08/34 (4 x LD50) on Day 0
- Lung histology on Day +19 post-challenge

Balb/c mice administered an intranasal dose of RD-Ad5 (3.2 x 108ifu) on Day -2 and challenged with influenza A/CA/04/2009 (3 x LD50) on Day 0. Cytokines in lung lavage were analyzed on Days 3 and 6; mean ±SD, p ≤ 0.05, **p ≤ 0.01 by ANOVA
Indications:
- Prevention of clinical worsening and hospitalization of ambulatory patients with early COVID-19
- Prevention of COVID-19 in individuals at high-risk of infection (known exposures)
- Potential first-line community protection against future strains of coronavirus and other pandemics
Mode of administration: Single dose, intranasal, with potential for self-administration
Storage and distribution: Stable at ambient temperatures for 3 or more months
Safety profile: Similar to placebo
- 96 community-based patients with fever, cough, or shortness of breath, with onset of symptoms within 48 hours, and a diagnosis of COVID-19 within 24 hours, will be randomized 1:1 to NasoVAX or placebo administered as a single 0.5 mL nasal spray on the day of diagnosis
- The study will consist of 3 cohorts of increasing age and risk for complications of COVID-19
- Primary efficacy endpoint
- Proportion of patients with clinical worsening, defined as a 4% decrease in pulse oxygen saturation (SpO2), or hospitalization
- Secondary endpoints
- Average decrease in resting SpO2
- Average increase in resting pulse rate
- Proportion of patients requiring oxygen supplementation and mechanical ventilation
- FDA agreed to allow Altimmune use its existing lot of RD-Ad5-based NasoVAX influenza vaccine for this trial so that it may be initiated quickly
