- Home
- Companies
- Centinel Spine
- Products
Centinel Spine products
Cervical
Evolving the Gold Standard in Cervical Spinal Fusion Procedures
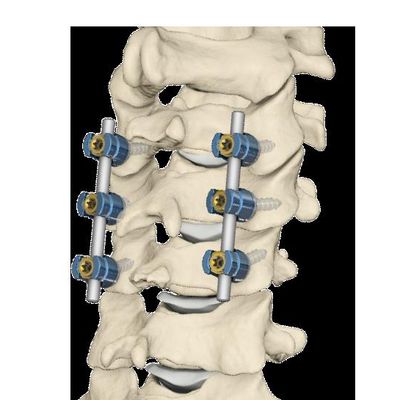
Introducing the First FDA cleared posterior cervical stabilization system specifically designed for use in either the cervical lateral masses or cervical-thoracic pedicles. Delivering the Results Surgeons Demand: Delivering gold standard clinical outcomes with a simple, comprehensive posterior cervical-thoracic stabilization system. Minimize tissue irritation for enhanced patient comfort with a low-profile screw and rod system, Simple rod placement resulting from friction stabilized tulip head, Streamlined surgical procedure performed with simple, low-volume instrument set
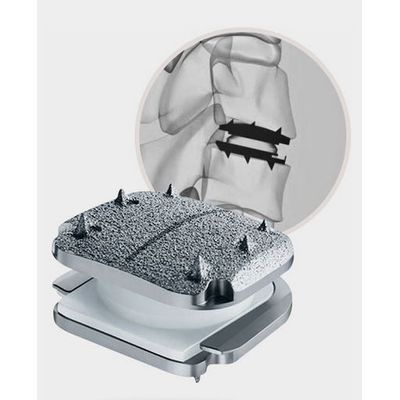
No-Profile® Anterior Cervical Integrated Interbody System
Anterior Cervical Integrated Interbody System
3D-printed porous titanium cervical interbody with 2-level clearance! lexible: FLX implants include a solid exoskeleton for added strength, along with a proprietary lattice scaffold designed to have a stiffness similar to bone. Reduced device stiffness is known to minimize stress-shielding and enhance the fusion process. Lucent: FLX implants include porous radiolucent sections with optimized density to reduce imaging artifacts—enhancing intra-operative visualization & post-operative fusion assessment compared to solid titanium implants. MatriX: FLX implants include a FUSE-THRU™ osteoconductive trabecular scaffold, bioengineered to allow the potential for bony on-growth and in-growth throughout the implant. This unique porous structure contains micro, macro, and nano structural characteristics designed to promote the fusion healing process by influencing cellular events related to the production of new bone.
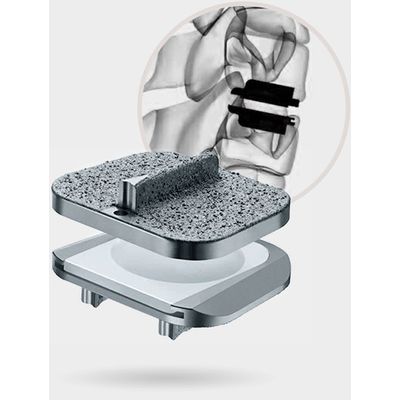
Prodisc - Anterior Cervical Total Disc Replacement
The most studied and clinically-proven total disc replacement technology in the world.
Anterior Cervical Total Disc Replacement
The most studied and clinically-proven total disc replacement technology in the world. Determined Safe & Effective for SCDD: The prodisc C Nova Total Disc Replacement is intended to replace a diseased and/or degenerated intervertebral disc of the cervical spine in patients with symptomatic cervical disc disease (SCDD). The prodisc C Nova Total Disc Replacement surgery is intended to: Significantly reduce pain by allowing for the removal of the diseased disc, Restore biomechanical stability, Restore normal disc height, Provide the potential for motion at the affected vertebral segment.