- Home
- Companies
- Tissue Genesis
- Products
Tissue Genesis products
Icellator - Enzyme-Derived Stem Cell Isolation System
The Icellator2 system set the standard, providing SVF cell yields in 65 minutes. Currently approved for use in The Bahamas and available in Japan. In the USA, clinical FDA-approved IDE studies using the Icellator® are underway for a variety of indications.
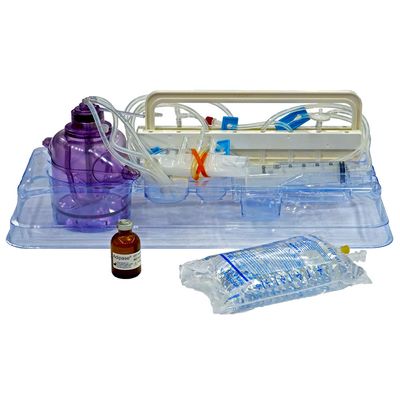
Icellator - Cell Icellation Kit
Sterile, single-use disposable kit using adipose tissue for high efficiency enzyme derived SVF isolation with Icellator2. Functionally closed system. Minimizes process and cell product variability. Temperature controlled digestion and separation. Class III approval for commercial use by the South Korean Ministry of Food and Drug Safety (MFDS). Approved for clinical use in South Korea & The Bahamas. In the USA, FDA-approved clinical IDE studies using the Icellator and Cell Icellation Kit are underway for multiple indications. Produced under ISO 13485 according to 21 CFR 820 (GMP).