- Home
- Companies
- Tissue Genesis
- Products
- Icellator - Model 2 - Cell Icellation ...
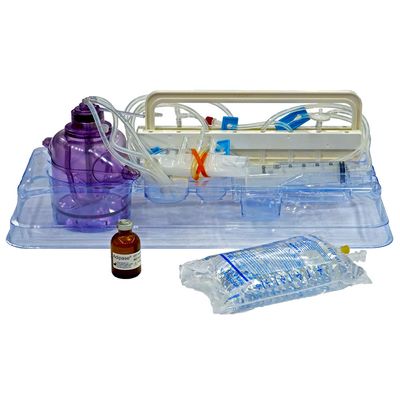
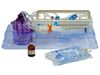
Icellator - Model 2 - Cell Icellation Kit
Sterile, single-use disposable kit using adipose tissue for high efficiency enzyme derived SVF isolation with Icellator2. Functionally closed system. Minimizes process and cell product variability. Temperature controlled digestion and separation. Class III approval for commercial use by the South Korean Ministry of Food and Drug Safety (MFDS). Approved for clinical use in South Korea & The Bahamas. In the USA, FDA-approved clinical IDE studies using the Icellator and Cell Icellation Kit are underway for multiple indications. Produced under ISO 13485 according to 21 CFR 820 (GMP).
THE ICELLATOR2 ICELLATION KIT BENEFITS
UNIQUE INTEGRATED DIGESTION, WASHING AND RESUSPENSION
- Injection port for addition of adipose tissue.
- Delivers final cell product to integrated syringe for immediate use.
- Sterile end-to-end cell isolation path.
- Proprietary Adipase® enzyme enables the highest possible yield of viable cells per ml of lipoaspirate.
- Automated addition of Adipase enzyme for temperature-controlled tissue and separation process leads to efficient SVF isolation1.
DELIVERS CELL PRODUCT READY FOR USE
- Delivers cell product into a syringe ready for immediate use.
- Minimizes sterile breaks and opportunity for contamination.
- Once loaded with a replacement Icellation kit, the Icellator2 is immediately ready for the next patient’s sample.
DESIGN AND AUTOMATION
- Gamma-irradiated, sterile polycarbonate cell separation and centrifugation chamber with double wall construction and optimized separation geometry.
- Transparent construction for real-time observation.
- Precision stainless cannulas for filling and aspiration.
- Luer injection port and cap for lipoaspirate loading.
- Motion Industries® precision high-rpm load bearing and nitrile pneumatic seal with resistive torque arm.
- Sterile fluid control polyallomer matrix assembly for controlling liquid flow during automated processing.
- Platinum silicon tubing assembly, Versapor® vents and custom solids filtration.
- Sterile ethylene vinyl acetate solution reservoir for wash buffer.
- 30 mL amber glass vial, aseptically-filled with lyophilized Adipase custom formulated for the digestion of adipose tissue.
SINGLE-USE STERILE PLASTIC KIT
- Produced under ISO 13485 and according to 21 CFR 820 (GMP).
- Manufactured in an ISO 7 Cleanroom.
- Sterilized by gamma irradiation.
- Nonpyrogenic.