- Home
- Software
- lithuania
- medical monitoring
- drug analysis
Refine by
Applications
Medical Monitoring Software Available In In Lithuania
70 software items found
by:Cority based inToronto, ONTARIO (CANADA)
The Clinic Visit Module is the central point of Medgate’s Occupational Health Software Suite. This robust and comprehensive module enables occupational health clinics to function at maximum productivity and effectively and easily manage and track all employee ...
by:Cority based inToronto, ONTARIO (CANADA)
The Practitioner Inbox Module in Medgate’s Occupational Health Software Suite is a one-stop access point for occupational health practitioners to review any activities requiring their ...
by:Cority based inToronto, ONTARIO (CANADA)
The Scheduling Module is a central hub to manage all clinic activities within Medgate’s Occupational Health Software Suite. This dynamic on-line appointment calendar enables organizations to run an efficient occupational health clinic through the automation of clinic scheduling ...
by:Noldus Information Technology bv based inWageningen, NETHERLANDS
Capture the full acoustic spectrum to process and analyze rodent ultrasonic calls and other animal ...
by:NantHealth, Inc. based inMorrisville, NORTH CAROLINA (USA)
Reimburse High-Quality, High-Value Autoimmune Care with Confidence. Providers don’t want to delay patient care. Payers don’t want to waste money on unwarranted, ineffective autoimmune care. Both want to improve clinical outcomes while ensuring healthcare dollars are spent ...
Manufactured by:Finapres Medical Systems based inEnschede, NETHERLANDS
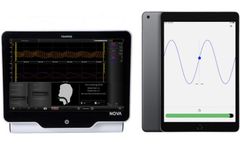
New GAT application enables standardization and quantification! In autonomic testing, standardized procedures are essential for reliable test results. Finapres Medical Systems developed a Guided Autonomic Testing (GAT) application as part of the Finapres® NOVA, which guides the operator and the patient through a series of autonomic test maneuvers. The GAT application consists of a graphical ...
Manufactured by:Finapres Medical Systems based inEnschede, NETHERLANDS
New GAT application enables standardization and quantification! In autonomic testing, standardized procedures are essential for reliable test results. Finapres Medical Systems developed a Guided Autonomic Testing (GAT) application as part of the Finapres® NOVA, which guides the operator and the patient through a series of autonomic test ...
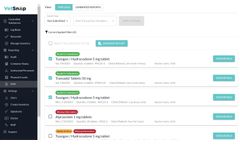
by:VetSnap Corporation based inThousand Oaks, CALIFORNIA (USA)
The VetSnap Prescription Monitoring Program (PMP) Digital Assistant is designed to enhance efficiency and accuracy in managing PMP compliance for in-clinic dispensed drugs. The assistant seamlessly integrates into existing VetSnap workflow systems, automating the collection and organization of required dispensing details. This automation reduces the likelihood of errors and missed submissions, ...
by:Endpoint Clinical based inWakefield, MASSACHUSETTS (USA)
DRIVE: Clinical Supply Management Solution. An enterprise-level solution, DRIVE, enables you to manage clinical supplies at the sponsor, depot, and site levels, providing your team with full visibility and traceability of inventory across all trials, inclusive or exclusive of the use of an ...
by:N-SIDE based inLouvain-La-Neuve, BELGIUM
End-to-end clinical trial supply chain visibility and optimization. Enable ambitious clinical plans through an optimal supply ...
by:Genuity Science based inBoston, MASSACHUSETTS (USA)
We are developing novel causal artificial intelligence and machine learning (AI/ML) and unconventional computing strategies to better understand human biology – and then apply these to deliver better medicine and improve health for people around the ...
by:N-SIDE based inLouvain-La-Neuve, BELGIUM
Optimize end-to-end production planning from DS to IMP. The N-SIDE Production App gives you unprecedented flexibility to optimize manufacturing for your clinical projects, from drug substance (DS) to drug product (DP) to investigational medicinal product (IMP). Use the Production App to build and maintain an optimal clinical production plan that accounts for your specific constraints. Respond ...
by:AWS Truepower, LLC based inAlbany, NEW YORK (USA)
Discover how our validated, FDA CFR Part 11 compliance training management system helps you comply with evolving FDA Regulations. Keep your workforce up to date. Medical device and pharmaceutical manufacturers trying to keep up with ever-changing regulations have a tough job. Medical technology advances quickly, and with it the need for compliance systems to support patient safety. To help you ...
by:Verana Health based inSan Francisco, CAMBODIA
Qdata™ by Verana Health are disease-specific, fit-for-purpose data modules designed to confidently drive business insights and rigorously inform research outcomes. Qdata spans three therapeutic areas-ophthalmology, neurology, and urology—and reflects deep patient journeys across robust demographics. Qdata helps to unlock quality research insights along the entire drug and medical ...
by:MasterControl, Inc based inSalt Lake City, UTAH (USA)
Small Medical Device Companies Can Now Manage and Automate their Design Control Process to Ensure Compliance with 21 CFR Part 820 with the Help of MasterControl's Design Control Software Systems at a Low Monthly Cost. Each manufactured medical device must be supported by appropriate documentation which demonstrates that its development followed the Food and Drug Administration's (FDA) design ...
by:Cresset Group based inCambridgeshire, UNITED KINGDOM
A single platform for ligand-based and structure-based drug design that enables research chemists to discover novel small molecules more efficiently and ...
by:SilcsBio, LLC based inBaltimore, MARYLAND (USA)
The CHARMM General Force Field program generates comprehensive parameters and topology information for a wide range of drug-like molecules, allowing their use in computer-aided drug design ...
by:CRScube Inc. based inSeoul, SOUTH KOREA
cubeIWRS® is a powerful solution that manages all aspects of a study's randomization, drug distribution, and delivery logistics. With cubeIWRS®, users have an intuitive, single platform that oversees randomization and drug supply management. ...
Manufactured by:SensitivE Audit based inStaten Island, NEW YORK (USA)
The LifeStream program was created specifically for sensitive Audit tools. Everything works the way you want and as regards the speed of information processing this software is many times superior to its competitors. The program's potential helps regularly update it, expand the database, increase the number of spectrograms, introduce new options, add features and capabilities. ...
by:CRScube Inc. based inSeoul, SOUTH KOREA
Pharmacovigilance requires a lot of resources such as personnel, processes, and management systems. cubeSAFETY® streamlines the Pharmacovigilance Reporting process; all the while being compliant with the regulations of regulatory agencies such as the FDA, EMA, CDE, MFDS, and ...