- Home
- Software
- europe
- medical monitoring
- drug analysis
Refine by
Medical Monitoring Software In Europe
36 software items found
by:Cority based inToronto, ONTARIO (CANADA)
The Clinic Visit Module is the central point of Medgate’s Occupational Health Software Suite. This robust and comprehensive module enables occupational health clinics to function at maximum productivity and effectively and easily manage and track all employee ...
by:Cority based inToronto, ONTARIO (CANADA)
The Practitioner Inbox Module in Medgate’s Occupational Health Software Suite is a one-stop access point for occupational health practitioners to review any activities requiring their ...
by:Cority based inToronto, ONTARIO (CANADA)
The Scheduling Module is a central hub to manage all clinic activities within Medgate’s Occupational Health Software Suite. This dynamic on-line appointment calendar enables organizations to run an efficient occupational health clinic through the automation of clinic scheduling ...
by:Noldus Information Technology bv based inWageningen, NETHERLANDS
Capture the full acoustic spectrum to process and analyze rodent ultrasonic calls and other animal ...
Manufactured by:Finapres Medical Systems based inEnschede, NETHERLANDS
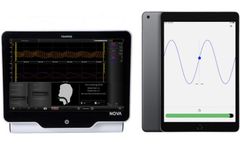
New GAT application enables standardization and quantification! In autonomic testing, standardized procedures are essential for reliable test results. Finapres Medical Systems developed a Guided Autonomic Testing (GAT) application as part of the Finapres® NOVA, which guides the operator and the patient through a series of autonomic test maneuvers. The GAT application consists of a graphical ...
Manufactured by:Finapres Medical Systems based inEnschede, NETHERLANDS
New GAT application enables standardization and quantification! In autonomic testing, standardized procedures are essential for reliable test results. Finapres Medical Systems developed a Guided Autonomic Testing (GAT) application as part of the Finapres® NOVA, which guides the operator and the patient through a series of autonomic test ...
by:Endpoint Clinical based inWakefield, MASSACHUSETTS (USA)
DRIVE: Clinical Supply Management Solution. An enterprise-level solution, DRIVE, enables you to manage clinical supplies at the sponsor, depot, and site levels, providing your team with full visibility and traceability of inventory across all trials, inclusive or exclusive of the use of an ...
by:N-SIDE based inLouvain-La-Neuve, BELGIUM
End-to-end clinical trial supply chain visibility and optimization. Enable ambitious clinical plans through an optimal supply ...
by:Genuity Science based inBoston, MASSACHUSETTS (USA)
We are developing novel causal artificial intelligence and machine learning (AI/ML) and unconventional computing strategies to better understand human biology – and then apply these to deliver better medicine and improve health for people around the ...
by:N-SIDE based inLouvain-La-Neuve, BELGIUM
Optimize end-to-end production planning from DS to IMP. The N-SIDE Production App gives you unprecedented flexibility to optimize manufacturing for your clinical projects, from drug substance (DS) to drug product (DP) to investigational medicinal product (IMP). Use the Production App to build and maintain an optimal clinical production plan that accounts for your specific constraints. Respond ...
by:MasterControl, Inc based inSalt Lake City, UTAH (USA)
Small Medical Device Companies Can Now Manage and Automate their Design Control Process to Ensure Compliance with 21 CFR Part 820 with the Help of MasterControl's Design Control Software Systems at a Low Monthly Cost. Each manufactured medical device must be supported by appropriate documentation which demonstrates that its development followed the Food and Drug Administration's (FDA) design ...
by:Cresset Group based inCambridgeshire, UNITED KINGDOM
A single platform for ligand-based and structure-based drug design that enables research chemists to discover novel small molecules more efficiently and ...
by:MasterControl, Inc based inSalt Lake City, UTAH (USA)
OOS Software to Automate Out of Specification (OOS) Processes to Comply with FDA CGMPs and 21 CFR Part 211 and Part 820 Regulations. In the FDA environment, specifications are essential in maintaining quality. CGMP regulations for finished pharmaceuticals (21 CFR Parts 210-211) and medical devices (21 CFR Part 820) require strict conformance to approved specifications which can be acheived easily ...
Manufactured by:Intelligence based inEschborn, GERMANY
Ontosight Explore enables the exploration, detection and building of various direct and in-direct connections between drug, target, pathway and disease. We leverage a biological network model using enhanced natural language processing, statistical scoring approach and entity normalization. Our self learning, life science ontology, has more than 20 million concepts and terms and brings together ...
by:Sapio Sciences based inBaltimore, MARYLAND (USA)
With our LIMS configurable technology and workflow templates, projects are completed in a fraction of the time and cost of standard LIMS ...
by:Qlucore AB based inLund, SWEDEN
Qlucore's clinical diagnostics solutions for multi-omics companion and precision diagnostics include AI-powered, disease-specific machine learning based classifier models and are combined with patient-friendly visualizations in a an easy-to-use and cost-effective software solution which integrates with a wide range of data generating techniques and instruments. ...
by:BrightInsight, Inc based inSan Jose, CALIFORNIA (USA)
The leading global regulated Digital Health platform for biopharma and ...
Manufactured by:Intelligence based inEschborn, GERMANY
The clinical trial prediction (CTP) engine empowers your decision-making: Predict the probability of clinical trial success, Evaluate-investment-decisions, Track clinical trial KPIs and identify critical path. Leverage AI to predict clinical trial outcomes: There are a lot of statistics about drug development – and all of them show that the process is inefficient and expensive: Pharma ...
Manufactured by:Intelligence based inEschborn, GERMANY
Identify the biomarkers best suited for your needs: Diagnose rare or ambiguous diseases, Group patients by endotype response for prognosis, Assess disease etiology to determine responsive patient group ...
by:Evolucare Technologies Shenzhen Co., Ltd based inFutian District, CHINA
The multidisciplinary solution for your hospital information ...