

- Home
- Companies
- Lineage Cell Therapeutics, Inc.
- Products
- OpRegen - Advanced Dry Age-Related ...
OpRegen - Advanced Dry Age-Related Macular Degeneration (Dry AMD)
On December 20, 2021, Lineage announced that it has entered into an exclusive worldwide collaboration and license agreement with Roche and Genentech, a member of the Roche Group, for the development and commercialization of OpRegen for the treatment of ocular disorders, including advanced dry age-related macular degeneration (dry AMD) with geographic atrophy (GA) in a transaction worth up to $670 million in addition to double digit royalties.
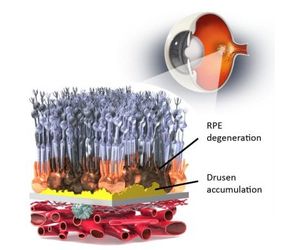
OpRegen® is currently being evaluated in a Phase 1/2a open-label, dose escalation safety and efficacy study of a single injection of human retinal pigment epithelium cells (RPE cells) derived from an established pluripotent cell line and transplanted subretinally in patients with advanced dry age-related macular degeneration (AMD) with geographic atrophy (GA). In November 2020, Lineage announced the completion of patient enrollment in this study.
The FDA has granted OpRegen with Fast Track Designations and, as such, is on an expedited regulatory path that includes the ability for increased interfacing with the FDA during clinical development and enhanced favorability for marketing approval.
In May 2022, data was presented at the 2022 Association for Research in Vision and Ophthalmology Annual Meeting. OpRegen Phase 1/2a clinical results support the potential for OpRegen to slow, stop or reverse disease progression in geographic atrophy secondary to age-related macular degeneration.
- 12-month primary endpoint data suggest OpRegen is well tolerated with an acceptable safety profile
- Preliminary evidence of visual function and outer retinal structure improvements observed in five Cohort 4 patients with GA and impaired vision
AMD affects more than 30 million people worldwide and approximately 1.6 million people are newly diagnosed annually in the U.S.1 It is a leading cause of vision loss in people over the age of 60 in the developed world. There are two forms of AMD: “wet AMD,” which affects only 10-15% of patients and “dry AMD,” which affects 85-90% of patients.2 Currently, there are only two FDA-approved therapies for the less common wet AMD, yet they constitute an estimated market of more than $10 billion.3
There are no FDA approved medical therapies for the 85 to 90 percent of AMD patients who suffer from the dry form. We believe one of the most promising future therapies for dry AMD is the replacement of the layer of damaged RPE cells that support and nourish the retina.
