- Home
- Companies
- Immune-Onc Therapeutics, Inc.
- Products
- Immune-Onc - Model IO-108 - ...
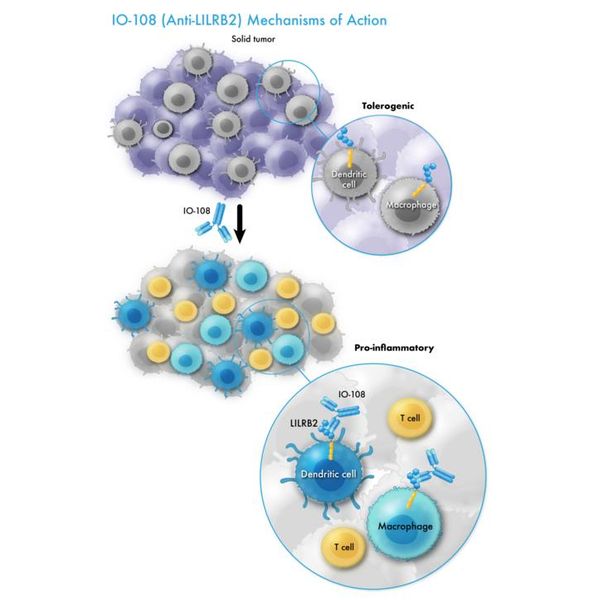
Immune-Onc - Model IO-108 - (Anti-LILRB2) Mechanisms of Action Program
Immune-Onc is evaluating IO-108, an antagonist antibody targeting the myeloid checkpoint, LILRB2 (also known as ILT4). The U.S. FDA has cleared the IND application for IO-108 as a potential therapy for solid tumors. The company expects to begin clinical trials with IO-108 in the second half of 2021.
LILRB2, also known as ILT4, is expressed mostly on myeloid cells, including monocytes, dendritic cells, macrophages, and neutrophils. In solid tumors, interaction of LILRB2 with tumor microenvironment (TME) relevant ligands, including HLA-G, ANGPTLs, SEMA4A, and CD1d, makes myeloid cells pro-tumorigenic (tolerating or promoting tumor growth) and promotes tumor immune evasion.
IO-108 is a fully human IgG4 monoclonal antibody with high affinity and specificity towards LILRB2 (also known as ILT4). It blocks the interaction of LILRB2 with multiple ligands that are involved in cancer-associated immune suppression, including HLA-G, ANGPTLs, SEMA4A and CD1d. Preclinical data presented at the 2020 Society for Immunotherapy of Cancer’s annual meeting and the 2022 American Association for Cancer Research annual meeting demonstrate that IO-108 functions as a myeloid checkpoint inhibitor and promotes innate and adaptive anti-cancer immunity.
In the ongoing Phase 1 study of IO-108 in adult patients with advanced or refractory solid tumors in the U.S. (NCT05054348), with a successful completion of dose escalation, IO-108 has been well tolerated with demonstrated clinical activity in multiple tumor types, both as a monotherapy and in combination with pembrolizumab, an anti-PD-1 antibody. This data enables the company to accelerate the global development of IO-108 in multiple expansion cohorts in select cancers as a monotherapy and in combination with T cell checkpoint inhibitors. The company has received the IND clearance in China and plans to evaluate IO-108 in solid tumors in China in 2H 2022.