

IntelliSelect - Antibody Repertoire Technology
We believe our IntelliSelect® Technology platforms reduce the risk of missing novel solutions and increase the quality and differentiation of antibodies, to identify product candidates with the most desirable drug-like properties.
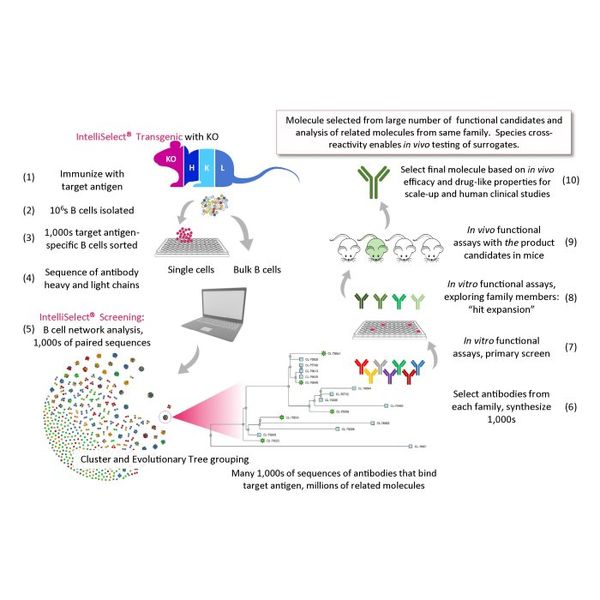
The IntelliSelect® Transgenics platforms are designed to generate fully-human monoclonal antibodies from highly-engineered strains of mice that have the complete constellation of human antibody building blocks in their genome. They are designed to produce fully human antibodies and we believe they are the most advanced human-antibody generation platforms developed.
Our IntelliSelect® Screening technology combines single-cell sequencing, genomics and proprietary bioinformatic algorithms to prioritize and select antibodies generated by our IntelliSelect® Transgenic platforms that have the most desirable drug-like properties.
Our IntelliSelect® Transgenic platforms utilize the immune system’s natural mechanisms to create fully-human antibodies that are mature or evolved to be highly specific for their target. We have achieved this by creating several strains of mice in which a complete set of human antibody genes and their landscape of surrounding human genomic DNA was carefully reconstructed in the mouse.
The human antibody gene loci in our IntelliSelect® Transgenic platforms mirror those in a human because we utilize the full expanse of the sequence found in humans.
Antibody development
The order and orientation of the genes that encode the antibodies, as well as the intergenic DNA, have an important regulatory function which we recreate in the mouse. As a result, the human loci can function as if they are operating in a human B cell, even though the human genes are present in mouse B cells.
When immunised with human targets or pathogenic organisms, Kymab`s mouse antibody platforms deliver a normal immune response and mature antibody drugs from their human building blocks. Because the human antibodies are produced through the normal and natural process of maturation, they evolve from molecules with good drug-like properties to drugs with exceptional potency, stability and solubility. Importantly, when these antibody genes are transferred into mammalian expression systems the recombinant molecules are reliably expressed at high levels.
When immunised with our target of choice, our platforms initiate tens of thousands of parallel antibody evolutionary processes in vivo, resulting in the creation of extensive family trees of affinity-matured human antibodies, which our IntelliSelect® Screening technology platform can then sequence, sort and evaluate.
Our modular antibody-discovery platforms can be modified further to support the discovery of highly differentiated antibodies and molecules with modes of action that would be unobtainable with other platforms. We invest in development of our platforms to facilitate specific drug-discovery efforts, such as target-locus knockouts and focusing of the repertoire by pre-setting the heavy or light chain to engineer bispecific antibodies. We have also generated mice with an enhanced average CDR3H length which will more effectively deliver antibodies against less-exposed epitopes.
We can further optimize our IntelliSelect® Transgenic platforms by inactivating the gene that encodes the protein that is the homolog of our selected drug target, also known as a knock-out, or KO.
Our gene inactivation capabilities allow our platforms to generate a greater diversity of antibodies that can bind to targets that have historically been difficult to reach. This allows us to isolate cross-reactive antibodies that target parts of antigens that are conserved between species.
Cross-reactivity also enables more efficient preclinical development of our product candidates because most animal efficacy models are rodent-based and therefore we can test the actual drug rather than a surrogate or mouse- equivalent using our platforms.
We are leveraging our IntelliSelect® platforms to develop bispecific antibodies, which can simultaneously bind to two cells or molecules. Targets may be located on two different cells or maybe two different molecules and a bispecific antibody brings those cells or molecules together. Bispecific antibodies can also bind two different targets on the same cell or bind two different soluble proteins, providing enhanced specificity or novel biological activity.
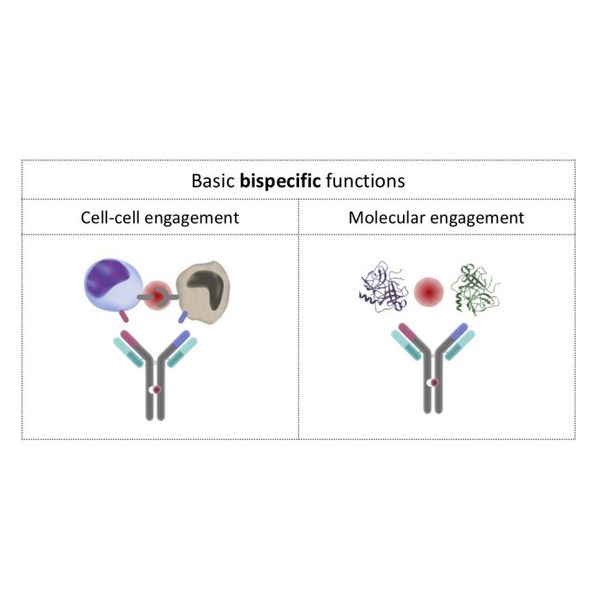
We believe that the characteristics of our platforms offer several advantages for the development of bispecific antibodies. Our bispecific antibodies are immunoglobulin G, or IgG, antibodies, with a common light chain and different heavy chains. To generate these antibodies, we conduct parallel immunizations with two antigens and then engineer a bespoke version of the transgenic platform to generate a diverse set of heavy chains. Functional screening identifies heavy chain pairs with balanced affinities for their targets and support robust purification of the bispecific antibodies for manufacturing purposes.
Two classes of molecule with therapeutic potential that are not currently addressed by antibody drugs are G-protein-coupled receptors and ion channels, largely because they display small epitopes not accessible to traditional antibodies and their sequence is often conserved and thus less likely to be antigenic.
We are able to address the second challenge because our unique technologies allow us to remove orthologous genes and hence to search for conserved epitopes.
To address the first, we have explored existing studies that show that human antibodies that are able penetrate past a heavily glycosylated surface to contact deep and conserved epitopes in viral proteins have long CDR3s. We have used this information to engineer our systems to more frequently deliver antibodies with longer-than-average CDR3 loops, which we believe are useful to address ion channels and G-protein-coupled receptors.
Kymab has developed a unique platform to discover antibodies that we anticipate will be able to modulate the activity of G-protein-coupled receptors, active sites of enzymes and pathogen antigens.
Both infected cells and, in some instances, cancer cells, display degraded internal proteins on their cell surface in the context of Major Histocompatibility Complex (MHC) proteins. Proteins of this type are recognised as self or non-self by T cell receptors on cytotoxic T cells. Kymab`s antibody discovery platforms have been used to isolate antibodies which recognise specific peptide-MHC complexes, providing access to a unique repertoire of new targets.
We discover and develop antibody therapeutics to build a strong portfolio of assets.
