

- Home
- Companies
- Biomex GmbH
- Products
- Model SCP-CMV-001 - CMV IgM ...
Model SCP-CMV-001 - CMV IgM Seroconversion Panel
The Biomex GmbH CMV IgM Seroconversion Panel consists of 15 members with each member containing 0.5 mL of human plasma. This panel illustrates the onset and decline of IgM and IgG Cytomegalovirus antibodies from one individual over a period of 99 days.
1. Intended Use
This Seroconversion Panel (SCP) is intended for standard testing by diagnostic manufacturers and researchers during assay development, evaluation, troubleshooting and post-marked surveillance of IgM/IgG antibody test systems and methods. Moreover, it serves as validation tool for diagnostic sensitivity, determination of analytical sensitivity, identification of cut-off values or to study the humoral immune response to this infection.
The plasma has been defibrinated and filtered. Sodium azide (0.095%) was added as preservative. All panel members are ready to use.
2. Storage and Stability
Store the SCP at -20°C to -80°C. Thaw samples at room temperature and mix gently by inversion before usage. Avoid foaming, contamination and repeated freeze and thaw cycles. After usage, return immediately to storage conditions.
3. Warnings and Precautions
Potentially infectious materials. Handle the product as if capable of transmitting infectious diseases. Do not pipette by mouth.
WARNING: contains sodium azide.
H302: Harmful if swallowed.
H413: May cause long lasting harmful effects to aquatic life.
Prevention:
P264: Wash hands thoroughly after handling.
P270: Do not eat, drink or smoke when using this product
P273: Avoid the release to the environment
Reaction:
P330: Rinse mouth.
P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
Disposal:
P501: Dispose of waste in accordance to applicable local or national regulations. Waste must be disposed in a secured manner.
4. Donor Information
All panel members have been tested and found negative/non-reactive for anti-HIV, anti-HCV,
HBsAg and Syphilis with FDA approved tests.
Donor profile
- Sex: Female
- Age: 20
- Ethnicity: Hispanic/Caucasian
- Residence: U.S. West Coast
- Children: yes
Suspected mode of transmission
- Mother-child interaction
Medical background
- No recent medication
- No recent illness or change in lifestyle
- No history of high risk behaviour
- No history of surgery
- No history of hepatitis or liver disease
- No history of blood transfusions
- No history of drug or alcohol abuse
5. Detection Methods
Each panel member is assayed for IgM and IgG antibodies with Abbott ARCHITECT®, Roche ELECSYS® and Diasorin LIAISON® test devices. Testing is performed with CE marked test.
6. Limitations and Restrictions
This panel is for Research Use Only and not intended for human or animal diagnostics, or for therapeutic purposes. Each laboratory has the responsibility to ascertain the suitability of the SCP for its particular application and to establish their own guidelines for interpretation of results. Data is provided for informational purposes only. The Biomex GmbH does not claim that others can duplicate these test results exactly.
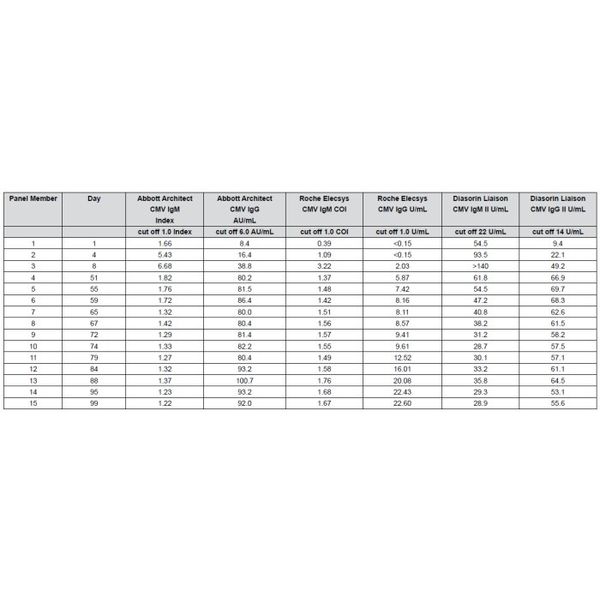
Assay information:
Abbott Architect CMV IgM assay; Lot: 52129LF00; Exp. Date: 13.04.2016; Test Date: 11.08.2015
Abbott Architect CMV IgG assay; Lot: 51027LF00; Exp. Date: 04.03.2016; Test Date: 11.08.2015
Roche Elecsys CMV IgM assay; Lot: 17091200
Roche Elecsys CMV IgG assay; Lot: 16971900
Diasorin Liaison CMV IgM II assay; Lot: 167019X/1
Diasorin Liaison CMV IgG II assay; Lot: 161021X/1
