

- Home
- Companies
- OSE Immunotherapeutics
- Products
- Model OSE-127/ S95011 - Humanized ...
Model OSE-127/ S95011 - Humanized Monoclonal Antibody
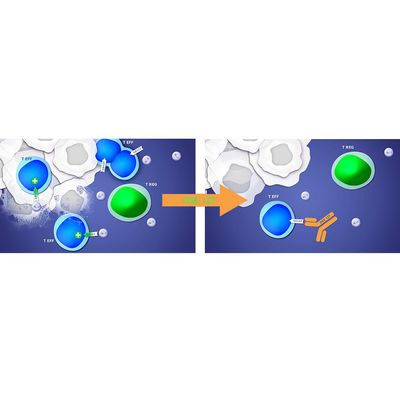
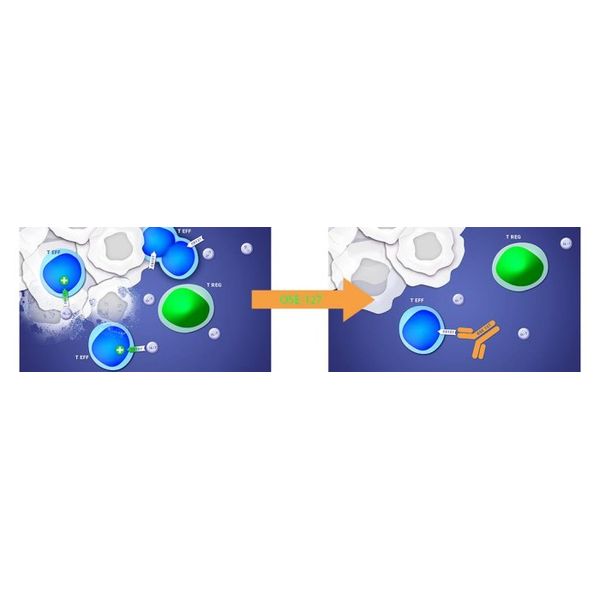
A humanized monoclonal antibody, is an antagonist of the alpha chain of the interleukine-7 receptor, CD127, present on T effector cells, thus down regulating the immune activity. Interleukin-7 (IL-7) is a cytokine that controls the proliferation, apoptosis and activation of CD4 and CD8 effector T-cells in humans.
OSE-127/S95011 is under an option license agreement signed with Servier in December 2016 to be developed up to the completion of a phase 2 clinical trial ongoing in Ulcerative Colitis, an autoimmune bowel disease; in parallel, OSE-127/S95011 is being developed in Sjögren’s syndrome, one of the most common chronic systemic autoimmune diseases, with an incidence of 60.82 per 100,000 people, according to a meta-analysis of epidemiological studies in Sjögren’s syndrome (Qin et al, 2015).
- Results from Phase 1 clinical study
- A Phase 2 study ongoing in Ulcerative Colitis
- A Phase 2 study ongoing in Sjögren’s syndrome sponsored by Servier
The Phase 1 showed a good safety and tolerability profile for OSE-127/S95011 in all 63 recruited healthy volunteers. All pharmacokinetic and pharmacodynamic parameters are consistent and demonstrate a dose-proportionality across the several dose-levels up to 10 mg/kg.
These results helped determine the dosing and administration schedule for the two independent Phase 2 trials ongoing with OSE-127/S95011:
- In Ulcerative Colitis (OSE Immunotherapeutics sponsor) : a randomized, double-blind Phase 2 clinical trial aiming at assessing the efficacy and the safety of OSE-127/S95011 versus placebo in patients with moderate to severe active ulcerative colitis who have previously failed or lost response or are intolerant to previous treatment(s).
ClinicalTrials.gov : NCT04882007 - In Sjögren’s Syndrome (Servier sponsor): a randomized, double-blind, placebo-controlled Phase 2 trial study evaluating the efficacy and safety of OSE-127/S95011. The primary endpoint is the change in ESSDAI total score (EULAR Sjogren’s Syndrome Disease activity Index), a physician-administered clinical index which has been validated to objectively assess systemic manifestations in Primary Sjögren’s Syndrome patients.
ClinicalTrials.gov : NCT04605978
