Refine by
Arterial Access Equipment Supplied In Usa
14 equipment items found
Manufactured by:Viant Medical based inFoxborough, MASSACHUSETTS (USA)
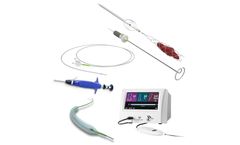
Complex interventional devices. Steerable, balloon, and RF ablation catheters. Closure devices (femoral arterial and venous access). Introducers for transradial artery access. ...
Manufactured by:TransLite LLC based inSugar Land, TEXAS (USA)
Finding and accessing veins and arteries on mice and rats has never been easier! Ideal for the Research market, Veinlite R® is specifically designed to simplify venous and arterial access on mice and rats. Veinlite R® is leading the way to better intravenous access in Research. It is simple to use, ...
Manufactured by:Prytime Medical Devices, Inc. based inBoerne, TEXAS (USA)
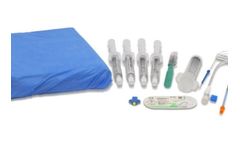
The REBOA Convenience Set is compatible with all Prytime Medical™ REBOA catheters and provides the clinician with the components needed for common femoral artery access, REBOA placement, and external fixation of the ...
Manufactured by:Prytime Medical Devices, Inc. based inBoerne, TEXAS (USA)
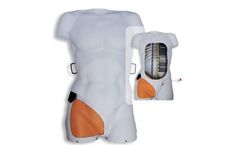
The Prytime Medical™ ARTI is designed with clinically accurate conditions and anatomy which are critical to arterial access and catheter placement. The ARTI system works with the entire family of Prytime ...
Manufactured by:TransLite LLC based inSugar Land, TEXAS (USA)
Finding and accessing veins and arteries on neonates has never been easier! Veinlite Neo is the only device on the market designed to help healthcare providers locate both veins and arteries in neonates. Veinlite NEO’s new form factor makes it easy, convenient and readily available for assisting with venous and arterial ...
Manufactured by:Simulab Corporation based inSeattle, WASHINGTON (USA)
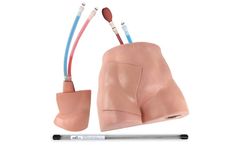
Simulab's FemoraLineMan System is an ultrasound-compatible femoral line vascular access trainer. The product offers an effective training solution for central venous or arterial access at the femoral site. ...
Manufactured by:Simulab Corporation based inSeattle, WASHINGTON (USA)
Simulab's FemoraLineMan Training Package is an ultrasound-compatible task trainer that offers an effective training solution for central venous or arterial access using the femoral site. This femoral line trainer uses the same patented technology as the highly acclaimed TraumaMan System and allows medical professionals to train using real-time ultrasound ...
Manufactured by:BIOMODEX based inQuincy, MASSACHUSETTS (USA)
Ischemic Stroke Cartridges, Multi-Use Type I/II/III Aortic Arches based on patient 3D rotational angiography, Right Femoral and Right & Left Radial Access, THROMBOTECH™ Hydrogel Synthetic Clot, Hard, Medium and Soft Textures, Lightweight, portable, plug-and-play station. BIOMODEX® EVIAS™ Plus is an ischemic stroke training and education solution designed for ...
Manufactured by:BIOMODEX based inQuincy, MASSACHUSETTS (USA)
Utilizes FDA cleared and CE marked software for segmentation of 3D RA or CTA, INVIVOTECH® is a proprietary* technology that integrates tissue biomechanics in 3D printed anatomical twins, Anatomical twin for intracranial aneurysm procedures includes vertebral artery access, ICA, distal vasculature and aneurysms, Contrast agent and X-ray compatible ...
Manufactured by:Shockwave Medical Inc. based inSanta Clara, CALIFORNIA (USA)
Quicker Cycle Time With 2x Faster Pulsing. 2X Faster Pulsing: Shockwave M5+ delivers the same amount of energy quicker with the same safety and efficacy profile as Shockwave ...
Manufactured by:Sonivate Medical based inPortland, OREGON (USA)
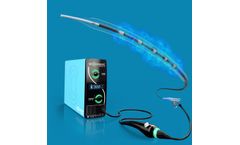
The initial 8.5 MHZ Linear Probe is unique with its patented design. It incorporates a revolutionary approach to micro-array architecture and connector cabling design and has produced a platform technology where the transducer is miniaturized and centered on a finger-mounted ...
Manufactured by:United Biologics, Inc. based inIrvine, CALIFORNIA (USA)
The Transcatheter Aortic Valve Replacement model is our transparent, versatile platform for training and product development. This vessel features two replaceable aortic valves, the left ventricle, and a full aorta down to the femoral arteries. Femoral access and modular heart valves allow for full simulation of transcatheter therapy scenarios and easy removal of ...
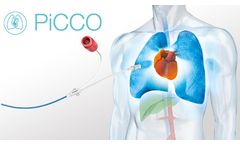
Manufactured by:Getinge AB based inGöteborg, SWEDEN
Cost-efficient tool for hemodynamic monitoring at the highest level. Monitoring physiological parameters is essential for the goal directed management of critical care patients. ...
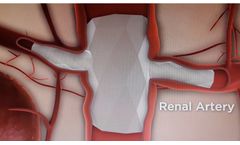
Manufactured by:Red Vascular Technology, LLC based inTampa, FLORIDA (USA)
Red Vascular’s non-modular, branched endoprosthesis represents an ideal solution for aortic aneurysmal disease with branch artery involvement. Its design and delivery through a single femoral artery site are superior to modular, fenestrated grafts currently used to treat complex aneurysms. Fenestration adds several complex steps to deployment of multiple grafts and leads to ...