Refine by
Blood Typing Equipment Supplied In Usa
42 equipment items found
Manufactured by:Access Biologicals LLC based inVista, CALIFORNIA (USA)
Human AB Serum lacks antibodies against the A and B blood type antigens, which prevents reaction with cultured cells and is recommended for use in biomedical applications. Human AB serum is regularly used for tissue engineering, transplantation and expansion of T-cells, cell therapy applications and cell line testing for PBMCs, bone marrow, CD9, CD3 and CD33. ...
Manufactured by:CD Bioparticles based inShirley, NEW YORK (USA)
Plain liposomes are extensively used as artificial cell models and for studying the interaction of various types of molecules such as peptides and proteins with the surface of the lipid membranes. Plain liposomes are also used as control formulations for many different types of drug encapsulated liposome formulations. Charged liposomes are also used in many ...
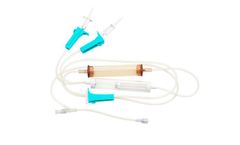
Manufactured by:Amsino International, Inc. based inPomona, CALIFORNIA (USA)
Y-Type Blood Set with 170 Micron Filter, Roller Clamps, Hand Pump, Slide Clamp, Roller Clamp, MicroClave Clear Needle-Free Y-Site ...
Manufactured by:Takeda Pharmaceutical Company Limited based inChuo-ku, JAPAN
Alogliptin benzoate (brand name: Nesina) is a treatment for hyperglycemia (high blood glucose) in Type 2 ...
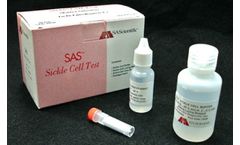
Manufactured by:SA Scientific, Ltd. based inSan Antonio, TEXAS (USA)
Add 20 ul whole blood to 2 ml of working sickle cell buffer. Read results within 30 ...
Manufactured by:Access Bio based inSomerset, NEW JERSEY (USA)
Recommended by the WHO during the Malaria Policy Advisory Meeting. G6PD deficiency is an X-linked recessive genetic disorder, resulting in no or low G6PD activity. G6PD deficienct patients are vulnerable to the spontaneous destruction of red blood cells when exposed to high oxidative stress. High oxidative stress may result from consumption of primaquine (antimalarial), aspirin, Fava beans, and ...
Manufactured by:Immucor based inNorcross, GEORGIA (US) (USA)
Reliably type red blood cells antigens beyond traditional ABO Blood Grouping ...
Manufactured by:Omron Healthcare, Inc based inHoofddorp, NETHERLANDS
OMRON EVOLV - The new, easy to use, All in One Upper Arm Blood Pressure Monitor. Take accurate readings in any position around the upper arm1 & track progress via ...
Manufactured by:Shenzhen AIVD Biotechnology Co. , LTD. based inCheektowaga, NEW YORK (USA)
Easy to carry, one-key operation; The instrument has a built-in battery for mobile detection; Automatically print and upload data; With ID card recognition and camera ...
Manufactured by:Drucker Diagnostics based inPort Matilda, PENNSYLVANIA (USA)
The drop-in replacement for the BD Sero-Fuge. Drucker Diagnostics worked closely with BD to create the SERO 12, a drop-in replacement for the discontinued Sero-Fuge. In fact, the SERO 12 uses the exact same rotor and accessories as the Sero-Fuge, so your existing serology processes can remain ...
Manufactured by:Access Bio based inSomerset, NEW JERSEY (USA)
The CareStart™ EZ HIV 1/2 is an in vitro RDT for the qualitative detection of antibodies to HIV type-1 and/or type-2 in serum, plasma, capillary whole blood or venous whole blood. The device is a single-use intended for healthcare professional use as an aid to diagnosis only – not for screening blood ...
Manufactured by:B Medical Systems based inHosingen, LUXEMBOURG
Blood Transport Box with 90 L | 3.18 cu ft gross ...
Manufactured by:B Medical Systems based inHosingen, LUXEMBOURG
Blood Transport Box with 20 L | 0.71 cu ft gross ...
Manufactured by:B Medical Systems based inHosingen, LUXEMBOURG
Blood Transport Box with 24 L | 0.85 cu ft gross ...
Manufactured by:B Medical Systems based inHosingen, LUXEMBOURG
Blood Transport Box with 44 L | 1.55 cu ft gross ...
Manufactured by:B Medical Systems based inHosingen, LUXEMBOURG
Blood Transport Box with 2.2 L | 0.08 cu ft gross ...
Manufactured by:B Medical Systems based inHosingen, LUXEMBOURG
Blood Transport Box with 8 L | 0.28 cu ft gross ...
Manufactured by:Vitalis, LLC based inNew York, NEW YORK (USA)
ER (extended-release) niacin currently has six FDA approved indications and has been prescribed to millions of patients in the U.S. alone (Jackevicius et al.). However, a well-known side effect of treating with ER niacin is Niacin Flush (Mills et al.). The Niacin Flush is a historical term for the medical syndrome of the “skin on fire” experience of these patients. Niacin Flush can be ...
Manufactured by:Moderna, Inc. based inCambridge, MASSACHUSETTS (USA)
At Moderna, we are delivering on the promise of mRNA science to create a new generation of transformative medicines for ...
Manufactured by:MP Biomedicals , LLC based inIrvine, CALIFORNIA (USA)
The Neonatal 17OHP ELISA Kit is intended for the measurement of 17-alpha hydroxyprogesterone (17OHP) in a whole blood ...