Show results for
Refine by
Bone Cavity Filler Equipment Supplied In Usa
15 equipment items found
Manufactured by:Zetagen Therapeutics Inc. based inSyracuse, NEW YORK (USA)
ZetaBase is an osteo-conductive and osteo-promotive therapy and is intended for the use as a bone void filler for bony voids or gaps that are not intrinsic to the stability of the bony structure. ZetaBase Bone Graft can be used as a bone graft ...
Manufactured by:Kinamed Incorporated based inCamarillo, CALIFORNIA (USA)
A simple set of instruments allows for precise creation of two compound wedges of bone. Transposing these wedges medializes the tubercle. ...
by:In2Bones Global based inMemphis, TENNESSEE (USA)
The i2b Resorbable Bead Kit is designed to create easy-to-use bio-resorbable beads for filling osseous defects. i2b® Resorbable Bead Kits allow surgeons to cast 100% pure synthetic resorbable bone void filler Beads in two sizes: 3.5 and 4.5mm. The beads provide a safe alternative to human or animal cadaver bone that completely eliminates the potential for disease transmission. The Mat ...
Manufactured by:OrthoPediatrics Corp. based inWarsaw, INDIANA (USA)
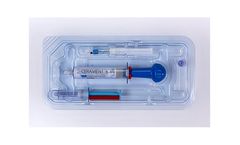
CERAMENT BONE VOID FILLER is an injectable, moldable and drillable synthetic bone void filler consisting of 40% hydroxyapatite, 60% calcium sulfate and the radio-contrast agent iohexol. The unique ratio of hydroxyapatite and calcium sulfate is designed to enable CERAMENT to resorb at the same rate that bone forms. Calcium sulfate acts as a resorbable carrier for hydroxyapatite, and hydroxyapatite ...
by:Molecular Matrix, Inc. based inWest Sacramento, CALIFORNIA (USA)
Osteo-P™ Bone Graft Substitute (BGS) is a non-mineralized, synthetic bone void filler consisting of a hyper-crosslinked carbohydrate polymer. It is highly porous, biocompatible, biodegradable, and osteoconductive. Osteo-P™ BGS is intended for the filling of bone defects created surgically or as a result of traumatic injury. When placed in direct contact with patient bone, ...
Manufactured by:Xenco Medical based inSan Diego, CALIFORNIA (USA)
The Sorrento™ Bone Graft Substitute is a robust, resorbable bone void filler to fill voids or gaps of the skeletal system in the extremities and pelvis not intrinsic to the stability of the bony structure. Pre-clinical animal studies have revealed its tremendous performance through early osteoblast activity and osteocyte formation with little fibrotic tissue. Sorrento™ Bone Graft ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
AMG Strips and Putty are unique and versatile bone void fillers comprised of partially demineralized cortical bone with putty-like handling characteristics. AMG can be rehydrated with BMA, PRP, whole blood, or ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
H-GENIN Crush-Mix is intended for use as a demineralized bone matrix for voids or gaps that are not intrinsic to the stability of the bony structure. Putty is indicated for the treatment of surgically created bone defects or bone defects created from traumatic injury. H-GENIN Crush-Mix provides a moldable consistency and can be mixed with blood or bone ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
H-GENIN Putty is intended for use as a demineralized bone matrix for voids or gaps that are not intrinsic to the stability of the bony structure. Putty is indicated for the treatment of surgically created bone defects or bone defects created from traumatic injury. H-GENIN Putty provides a moldable consistency and can be mixed with blood or bone ...
Manufactured by:VIVEX Biologics, Inc. based inMiami, FLORIDA (USA)
VIA DBM Plus™ is a demineralized bone matrix putty containing cortical bone chips and indicated for use in the extremities, posterolateral spine, and pelvis as a bone void filler or for treatment of osseous ...
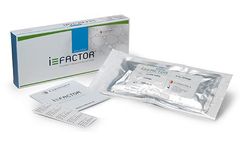
Manufactured by:Cerapedics, Inc. based inWestminster, COLORADO (USA)
i-FACTOR Bone Graft products are intended to replace or augment the use of autologous bone commonly utilized in spine, trauma, and orthopedic procedures such as: Spinal fusion incorporating interbody fusion devices; Treatment of non-union or traumatic fresh fractures; Joint reconstruction requiring a bone void filler. i-FACTOR Bone Graft products are sterilized after packaging (terminally ...
Manufactured by:EnColl Corporation based inFremont, CALIFORNIA (USA)
Over 100 years of research, experimentation and use has demonstrated that calcium sulphate enhances bone regeneration and is totally biocompatible and and resorbable. At the same token, high purity type-I collagen DMBM (xenograft) also is essential for tissue regeneration and remodeling. Osseomold consists of both materials admixed in proper proportions to give the best biological and mechanical ...
Manufactured by:EnColl Corporation based inFremont, CALIFORNIA (USA)
High purity Type-I collagen derived from bone is essential for tissue regeneration and remodeling in any osseous defect. Osseograft / DMBM (xenograft) is one such de-mineralized bone derived Type-I collagen for bone void filling ...
Manufactured by:Parametrics Medical based inLeander, TEXAS (USA)
Synthetic bone void fillers are safe, biocompatible, and easy to use. These resorbable biomaterials are based on nano-crystalline hydroxyapatite (HA) and purified collagen technologies. These products are engineered to optimize the resorption rate. Our granules are composed of 60% HA and 40% β-Tricalcium Phosphate (TCP). The formulation provides optimal osteoconduction, and the composition ...
by:NuShores Biosciences LLC based inLittle Rock, ARKANSAS (USA)
Today there is no satisfactory solution for major bone injuries caused by accidents, warfare and cancer, especially complex and large segmental bone loss. Our NuCress™ bone scaffold technology has been shown to promote healing of large segmental bone breaks (>2.5 cm) tested in large and small animals (goats, mice) – with no infections, inflammation, rejection or adverse bone ...