Refine by
Coronary Products Equipment & Supplies
12 equipment items found
Manufactured by:QT Vascular Ltd. based in, SINGAPORE
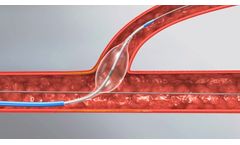
The Glider PTCA Balloon Catheter is the only torqueable angioplasty balloon catheter. Combined with its unique balloon shape and state of the art tip technology, the Glider™ is designed to cross through tight lesions and stent struts even in conditions where other balloons are challenged. The unique design of the Glider™ provides physicians with a powerful tool in managing the ...
Manufactured by:QT Vascular Ltd. based in, SINGAPORE
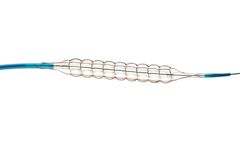
The control you need to create a predictable, uniform dilatation. Chocolate is designed to reduce vessel trauma by providing a balloon inflation that is*: Predictable, Controlled, ...
Manufactured by:Medinol based inTel Aviv, ISRAEL
Medinol’s patented Flexx² technology is a game-changer in coronary deliverability, especially in tough anatomies. Flexx² is based on a tapered spring tip designed to eliminate tip flare out and buckling while enabling simultaneous flexibility and pushability. The entire coronary product line of Medinol builds on Flexx² ...
Manufactured by:United Biologics, Inc. based inIrvine, CALIFORNIA (USA)
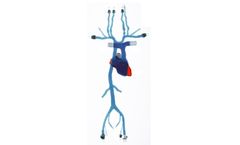
The Transseptal Model is our transparent, compliant model featuring access across the atrial septum, between the left and right atria. This model includes external/internal jugulars down to the iliacs, and a complete structural heart including the coronary sinus. ...
Manufactured by:ACIST Medical Systems based inEden Prairie, MINNESOTA (USA)
This catheter delivers more flexibility than before and has a streamlined crossing profile to access complex lesions. Navvus II and its predecessor, Navvus, are the only coronary physiology products that allow the use of your choice of 0.014” guidewires. This market distinction may enable faster, more efficient diagnostic and post-PCI physiological ...
Manufactured by:ACIST Medical Systems based inEden Prairie, MINNESOTA (USA)
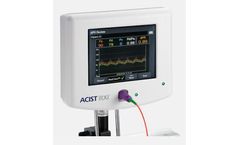
Non-hyperemic index for coronary physiology. ACIST diastolic pressure ratio (dPR), using the ACIST RXi Rapid Exchange System and Navvus MicroCatheter, provides a non-hyperemic alternative for physiological assessment of coronary ...
Manufactured by:Wellinq based inLeek, NETHERLANDS
High quality Angiographic catheter that provides reliable kink-free passage of tortuous anatomy and calcified lesions while keeping optimal torque control in the coronary ...
Manufactured by:Boston Scientific based inMarlborough, MASSACHUSETTS (USA)
CHANNEL Steerable Sheath is designed with a strong, steerable shaft to facilitate placement of devices in peripheral and coronary ...
Manufactured by:Genesee BioMedical, Inc. based inDenver, COLORADO (USA)
Multipurpose Retractor for valve repair, valve replacement and on or off-pump coronary artery ...
Manufactured by:JenaValve Technology, Inc. based inIrvine, CALIFORNIA (USA)
Nitinol Support Frame: Self-Expanding Design – Available in (3) Sizes. Large Cells for Coronary ...
Manufactured by:Baymed Inovative Health Products Inc. based inMerkez/Kilis, TURKEY
Baymed® disposable cardiovascular drapes provide the strength, versatility and efficiency needed for the critical demands of cardiovascular surgery. Baymed® disposable cardiovascular drapes, made with heavy-duty fabric and Advanced Bayteks® Reinforcement fabric for fluid management in fenestrations, may eliminate the need for costly drape layering. Baymed® disposable drapes for ...
Manufactured by:Vascudyne, Inc. based inStillwater, MINNESOTA (USA)
Synthetic vascular grafts currently used in clinic have poor long term outcomes and low patency rates. Hyperplasia, calcification, foreign body immune response, infection, and atheroma formation are commonly encountered limiting the use of synthetic grafts, with none available for small diameter applications like coronary ...