- Home
- Equipment
- north america
- detecting diseases
Refine by
Detecting Diseases Equipment Supplied In North America
28 equipment items found
Manufactured by:Diopsys, Inc. based in, NEW JERSEY (USA)
The Diopsys® ffERG (full field electroretinography) module includes several important protocols to help you detect disease early and manage patient care. Understanding how your patient is responding to treatment is important. Is their visual function improving? Is their disease progressing? Diopsys® ffERG / Flicker vision tests provide ...
Manufactured by:Optos, Inc. based inMarlborough, MASSACHUSETTS (USA)
Daytona produces a 200° single-capture optomap retinal image of unrivaled clarity in less than ½ second. This fast, easy, patient-friendly, ultra-widefield imaging technology was designed for healthy eye screening and has been shown to improve practice flow and patient engagement. ...
Manufactured by:Midmark Corporation based inVersailles, OHIO (USA)
As healthcare continues to change, there has been an increased focus on improving the patient experience and clinical outcomes. We are learning that subtle differences in technology can have a major impact on early detection of disease—starting at the ...
Manufactured by:Biomedix based inSaint Paul, MINNESOTA (USA)
NeuroMetrix is a leading provider of diagnostic technology for the identification of peripheral neuropathy. DPNCheck is a device designed to evaluate sural nerve conduction through an objective test that is easy to perform, and provides accurate results. The analysis of sural nerve conduction provides a standard biomarker for peripheral neuropathy, with technology that is accurate in the ...
Manufactured by:Entero Therapeutics, Inc. based inBoca Raton, FLORIDA (USA)
A majority of individuals with CD do not recover well under a GFD. Furthermore, there is still no effective means for monitoring the intestinal health of a recovering celiac other than an invasive biopsy and even that method is prone to interpretive analysis. This problem is particularly acute in the United States where recent studies have shown that more than 50% of adult celiac patients have ...
Manufactured by:Becton Dickinson and Company (BD) based inFranklin Lakes, NEW JERSEY (USA)
The BD SurePath™ liquid-based Pap test provides advantages over conventional Pap smears and other liquid-based cytology (LBC) tests, resulting in greater disease ...
Manufactured by:Guardant Health, Inc. based inRedwood City, CALIFORNIA (USA)
Our Guardant Reveal™ test is the first blood-only test that detects residual and recurrent disease in two weeks, without the need for a tissue biopsy. The test detects circulating tumor DNA (ctDNA) in blood after surgery to identify patients with residual disease who may benefit most from adjuvant therapy. ...
by:AptaMatrix, Inc. based inSyracuse, NEW YORK (USA)
Aptamers are DNA/RNA molecules that have affinities for their targets similar to antibodies. Aptamers have shown great potential to replace antibodies in biosensors, point-of-care diagnostics, therapeutics, and all of the areas currently dominated by antibodies. Once aptamers have been identified, they can be produced for less than 10% of the cost of antibodies. The commercial value of antibodies ...
by:ni2o, Inc. based inProvidence, RHODE ISLAND (USA)
Countering Communicable Diseases with our Five-Minute Test. Worldwide Testing Challenges: COVID-19 has opened our eyes to the many communities who don’t have adequate disease detection technology. Ni2o’s RapidTest instrument will allow for a variety of diseases to be detected from samples collected ...
Manufactured by:Nucleix Ltd. based inSan Diego, CALIFORNIA (USA)
Most patients are currently diagnosed with late-stage cancer, though research has shown 5-year survival rates are up to 10 times greater when disease is detected early. Annual imaging (low-dose CT scans) is recommended for screening individuals with highest risk, but less than 10% of those eligible adhere to guidelines2. Delivering an effective screening option ...
Manufactured by:Eko Devices, Inc. based inOakland, CALIFORNIA (USA)
Transform the stethoscope you already own and enhance auscultation through amplification, active noise cancellation, wireless listening, and Eko ...
Distributed by:Alban Scientific, Inc. based inSt. Louis, MISSOURI (USA)
The BD Veritor™ Plus System offers convenient point-of-care testing for SARS-CoV-2*, Influenza A+B, Group A Streptococcus, and respiratory syncytial virus and combines rapid, reliable results with ease of implementation and simple workflow ...
Manufactured by:C2N Diagnostics based inSt. Louis, MISSOURI (USA)
The PrecivityAD™ test is an innovative new blood test intended for use in patients with cognitive impairment. The test aims to help physicians better determine the presence or absence of amyloid plaques in the brain, a hallmark sign of Alzheimer’s ...
Manufactured by:Digital Diagnostics Inc. based inCoralville, IOWA (USA)
IDx-DR is an AI diagnostic system that autonomously diagnoses patients for diabetic retinopathy (including macular ...
Manufactured by:Eko Devices, Inc. based inOakland, CALIFORNIA (USA)
The 3M Littmann CORE Digital Stethoscope features the outstanding acoustics, comfort and quality of a Littmann cardiology-grade stethoscope enhanced by powerful digital ...
by:NeoGenomics Laboratories based inFort Myers, FLORIDA (USA)
RaDaR™ is a personalized, multi-tumor liquid biopsy assay that can track a set of up to 48 tumor-specific variants in a patient using a liquid biopsy with exceptional sensitivity. This allows RaDaR™ to detect traces of molecular residual disease (MRD) in a patient following surgery or other treatment for cancer, enabling early ...
by:BillionToOne Inc. based inMenlo Park, CALIFORNIA (USA)
Our molecular counting platform can help quantify nearly undetectable DNA changes, unlocking improvements to non-invasive prenatal screening and liquid ...
Distributed by:Labex Canada Inc. based in, ONTARIO (CANADA)
In humans, they are known to be involved mainly in respiratory infections ranging from the common colds to more severe diseases such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). The recently discovered coronavirus, SARS-CoV-2, causes coronavirus disease COVID-19, a significant global health problem. IgM antibodies ...
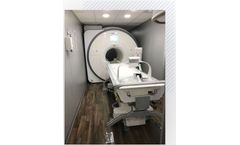
Manufactured by:Shared Imaging based inDowners Grove, ILLINOIS (USA)
MRI technology is a non-invasive imaging method that generates detailed three-dimensional anatomical images without the use of ionizing radiation, making it ideal for disease detection, diagnosis, and treatment monitoring. It excels in imaging non-bony or soft tissues such as the brain, spinal cord, nerves, muscles, ligaments, and tendons, offering superior ...
Manufactured by:Kailos Genetics, LLC. based inHuntsville, ALABAMA (USA)
Kailos Iota combines TargetRich, our foundational genetic enrichment technology, with next-generation sequencing and statistical machine learning that results in a highly specific cancer assay of multiple solid tumors at all stages. The Iota assay detects methylation differences in a person’s blood sample, the superior choice for early disease ...