Refine by
In Vivo Testing Equipment & Supplies In In Vietnam
15 equipment items found
Manufactured by:Life Spine, Inc. based inHuntley, ILLINOIS (USA)
Osteo-Link has been engineered and processed to deliver the highest level of osteoinductivity, according to in-vivo test results. Available in putty, strips, cubes, and flowable for a variety of ...
Manufactured by:InSphero AG based inSchlieren, SWITZERLAND
An in vitro PDX model with in vivo-like heterogeneity. 3D InSight™ Tumor Microtissues from Patient-Derived Xenografts (PDX) are custom assay-ready 3D in vitro tumor models engineered to reflect complex tumor biology without the use of artificial matrices. Ideal for early stage cancer drug efficacy testing and screening appliciations, these advanced cancer ...
Manufactured by:W. L. Gore & Associates, Inc. based inNewark, DELAWARE (USA)
Addressing the problem of thrombus formation on the luminal surface of a vascular graft is a challenge faced by all vascular surgeons. The GORE PROPATEN Vascular Graft is specifically designed for those vascular procedures in which the risk of acute graft thrombotic failure is of clinical ...
Manufactured by:W. L. Gore & Associates, Inc. based inNewark, DELAWARE (USA)
Addressing the problem of thrombus formation on the luminal surface of a vascular graft is a challenge faced by all vascular surgeons. The GORE PROPATEN Vascular Graft is specifically designed for those vascular procedures in which the risk of acute graft thrombotic failure is of clinical ...
Manufactured by:Alliance Spine based inSan Antonio, TEXAS (USA)
Promote OsteoStrip is a pliable and absorbable cancellous bone graft that also exhibits osteoconductive and osteoinductive properties. These characteristics used as a homologous tool for bone grafting in spinal fusions, non-unions, and bone fractures. OsteoStrip is processed using a next generation proprietary processing method that maintains the interconnected structure of trabecular ...
Manufactured by:Bonegraft Biyolojik Malzemeler San. VE TIC. A.S. based inIZMIR, TURKEY
Implants are supplied sterile and are CE marked. Class III Medical Device according to 93/42/EC, medical device directive. Biocompatibility tests (in vitro and in vivo), biodegradation tests, bioburden, and stylerity were applied to the products. Implants are used for temporary fixation of bone. Patellar tendon bone and soft tissue grafts in the ...
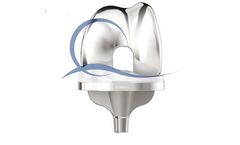
Manufactured by:Medacta International based inCastel San Pietro, SWITZERLAND
Patient expectations are not as well fulfilled by TKA as by total hip replacement with fewer knee patients achieving a “forgotten joint” replacement. Studies show that around 20% of TKA patients are not satisfied[1, 2, 3]. Excessive A/P motion may result in anterior knee pain and continued swelling. In many P/S designs, the stabilizing mechanism only engages after 70-80° of ...
Manufactured by:Advanced Microdevices Pvt. Ltd. (mdi) based inAmbala Cantt, INDIA
mdi AseptiCap KS are specially designed filters for process development and formulation development labs with identical materials of construction to larger capsule and cartridge filters for easy scale up. The 0.1µm rated PES membrane is validated for quick and efficient mycoplasma removal with enhanced throughtputs. 50mm Inline capsule filter is a specially vented device for use with ...
Manufactured by:Inovotion Sas based inLa Tronche, FRANCE
INOVOTION has developed a unique, highly sensitive and reproducible assay. It is applicable to all preclinical oncology and immuno-oncology drug discovery programs, and monitors the growth and metastatic dissemination of human tumor ...
Manufactured by:Advanced Microdevices Pvt. Ltd. (mdi) based inAmbala Cantt, INDIA
mdi Aseptisure KS 0.1µm double layer PES membrane mini cartridge filters are validated for mycoplasma removal and are used for sterile media filtration in mammalian cell culture. The upstream PES membrane layer protects the downstream PES membrane layer from premature clogging. The membrane pore structure is specially designed to give high throughputs, thus resulting in better ...
Manufactured by:Advanced Microdevices Pvt. Ltd. (mdi) based inAmbala Cantt, INDIA
mdi AseptiSure KS 0.1µm double layer PES membrane cartridge filters are validated for mycoplasma removal and are used for sterile media filtration in mammalian cell culture. The upstream PES membrane layer protects the downstream PES membrane layer from premature clogging. The membrane pore structure is specially designed to give high throughputs, thus resulting in better ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
AZD1080 is a potent and selective GSK3 inhibitor. AZD1080 inhibits recombinant human GSK3α and GSK3β with pKi (IC50) of 8.2 (6.9 nM) and 7.5 (31 nM), ...
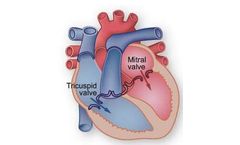
Manufactured by:Novostia SA based inEpalinges, SWITZERLAND
Your heart is the most important muscular organ in your body. It is a two-sided pump that beats about 100,000 times a day (more than 2 billion times in an average lifetime) to keep your blood circulation going. The right side of the heart pumps blood through the lungs where it picks up oxygen. The left side of the heart receives the blood containing oxygen and pumps the blood to the rest of your ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Entinostat is an oral and selective class I HDAC inhibitor, with IC50s of 243 nM, 453 nM, and 248 nM for HDAC1, HDAC2, and HDAC3, ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Ipatasertib dihydrochloride (GDC-0068 dihydrochloride) is a highly selective and ATP-competitive pan-Akt inhibitor with IC50s of 5, 18 and 8 nM for Akt1, Akt2 and Akt3, ...