- Home
- Equipment
- north korea
- intravenous solution
Refine by
Intravenous Solution Equipment & Supplies In North Korea
13 equipment items found
Manufactured by:B. Braun Medical Inc. based inBethlehem, PENNSYLVANIA (USA)
Dextrose and Sodium Chloride Injections USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents. These products are intended for intravenous administration. These intravenous solutions are indicated for use in adults and pediatric patients as sources of electrolytes, calories and water for hydration. Not made with ...
Manufactured by:B. Braun Medical Inc. based inBethlehem, PENNSYLVANIA (USA)
Dextrose and Sodium Chloride Injections USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents. These products are intended for intravenous administration. These intravenous solutions are indicated for use in adults and pediatric patients as sources of electrolytes, calories and water for hydration. Not made with ...
Manufactured by:B. Braun Medical Inc. based inBethlehem, PENNSYLVANIA (USA)
Dextrose and Sodium Chloride Injections USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents. These products are intended for intravenous administration. These intravenous solutions are indicated for use in adults and pediatric patients as sources of electrolytes, calories and water for hydration. Not made with ...
Manufactured by:B. Braun Medical Inc. based inBethlehem, PENNSYLVANIA (USA)
Dextrose and Sodium Chloride Injections USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents. These products are intended for intravenous administration. These intravenous solutions are indicated for use in adults and pediatric patients as sources of electrolytes, calories and water for hydration. Not made with ...
Manufactured by:B. Braun Medical Inc. based inBethlehem, PENNSYLVANIA (USA)
Dextrose and Sodium Chloride Injections USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents. These products are intended for intravenous administration. These intravenous solutions are indicated for use in adults and pediatric patients as sources of electrolytes, calories and water for hydration. Not made with ...
Manufactured by:B. Braun Medical Inc. based inBethlehem, PENNSYLVANIA (USA)
Dextrose Injections USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents. These products are intended for intravenous administration. These intravenous solutions are indicated for use in adults and pediatric patients as sources of calories and water for ...
Manufactured by:B. Braun Medical Inc. based inBethlehem, PENNSYLVANIA (USA)
Dextrose Injections USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents. These products are intended for intravenous administration. These intravenous solutions are indicated for use in adults and pediatric patients as sources of calories and water for ...
Manufactured by:Brevetti Angela S.r.l. based inArzignano, ITALY
It offers flexibility with modular moulds adaptable to various designs and materials such as PE, PP, and HDPE. Its compact size reduces clean room requirements, and it allows for configurations like implementable capping solutions using Eurocap and Ecohead designs. Essential applications include intravenous solutions, irrigation, sodium chloride, ...
Manufactured by:Shanghai IVEN Pharmatech Engineering Co., Ltd. based inShanghai, CHINA
The Non-PVC Soft Bag IV Solution Production Line is designed to streamline the production of intravenous solution bags using advanced technology. This system efficiently handles processes like film feeding, printing, bag making, filling, and sealing, all in one integrated machine. It supports a range of bag volumes from 50ml to 5000ml and caters ...
Manufactured by:CSL Behring based inKing of Prussia, PENNSYLVANIA (USA)
Intragam P is a sterile, preservative-free solution containing 6 g of human protein in each 100 mL. At least 98% of the protein has the electrophoretic mobility of immunoglobulin G (IgG). At least 90% of the protein is IgG monomer and ...
Manufactured by:B. Braun Medical Inc. based inBethlehem, PENNSYLVANIA (USA)
Isolyte P in 5% Dextrose is sterile, nonpyrogenic, and contains no bacteriostatic or antimicrobial agents or added buffers. This product is intended for intravenous administration. This solution is indicated for use in adults as a source of electrolytes, calories and water for hydration, and as an alkalinizing agent. Not made with latex, PVC or DEHP. ...
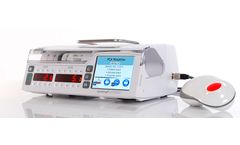
Manufactured by:Arcomed AG based inKloten, SWITZERLAND
Why not involve the patient while s/he is awake in decisions about bolus drug delivery, based upon his/her pain perception? Consideration of this factor whilst maintaining the highest safety standards has been our priority in the development of our PCA/PCEA/PCS ...
Manufactured by:Automatic Liquid Packaging Solutions LLC (ALPS) based inArlington Heights, ILLINOIS (USA)
The ALPS Hybrid 700 Series Machine is designed for advanced Aseptic Blow/Fill/Seal (BFS) and Form/Fill/Seal (FFS) applications. Developed with 43 years of experience, these machines incorporate Electric Servo Drives and Hydraulics to optimize packaging processes. They accommodate a range of container sizes from 0.1mL to 2000mL, providing versatile solutions for various products. The machine ...