Refine by
Iv Catheters Equipment Supplied In Usa
25 equipment items found
Manufactured by:Retractable Technologies, Inc. (RTI) based inLittle Elm, TEXAS (USA)
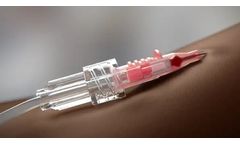
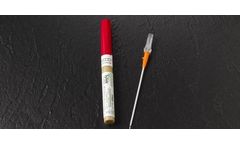
The VanishPoint safety IV catheter utilizes patented automated retraction technology similar to that of the VanishPoint syringe and blood collection tube holder. It is easy to use and allows for one-handed venipuncture. It contains an integrated safety mechanism that, when activated, automatically retracts the introducer needle, which remains safely retracted ...
Manufactured by:Optinova based inMariehamn, Åland, FINLAND
Optinfusion™ IV Catheter Tubing TPU 2.0 is made with a size range of 14-26 G. Optinova offers striped tubing with 2-6 radiopaque stripes. Barium sulfate (BaSO4) up to 40% is used for filler, yet we can accommodate to customer ...
Manufactured by:S2S Global based inCharlotte, NORTH CAROLINA (USA)
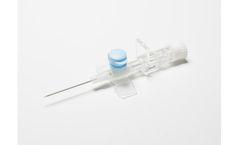
Our ClearSafe Comfort® Line in the PremierPro™ by Medsource Family of Catheters provides you the latest technology without having to sacrifice your preferred safety style. Select from a range of gauges and lengths that offer you the best in Safety and Blood Control. Our slide-activated safety features also help reduce the risk of accidental needlestick injuries. Each catheter is ...
Manufactured by:Optinova based inMariehamn, Åland, FINLAND
Optinova is the proud owner of the most reliable IV Catheter Tubing solutions in the market. Optinova’s Optinfusion™ IV Catheter tubing is made from TPU, FEP and ...
Manufactured by:S2S Global based inCharlotte, NORTH CAROLINA (USA)
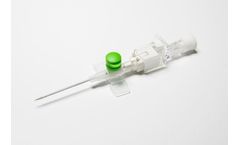
Our NanoCath TrueSafe Comfort® Line in the PremierPro™ by Medsource Family of Catheters provides you the latest technology for your tiniest patient. We offer 24G and 26G with two lengths so that you can have better chance of success your first try. Our push button activated safety features also help reduce the risk of accidental needlesticks injuries. Each catheter is equipped with our ...
Manufactured by:Medsource Labs based inChanhassen, MINNESOTA (USA)
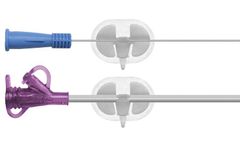
Blood Control Active: Check Valve Technology. Our patent-pending Active Check Valve inhibits the flow of blood during IV cannulation, engineered to protect healthcare professionals and their patients from exposure to bloodborne pathogens. Our innovative Blood Control IV catheters last for 5 years, whereas comparable products have a limited 2-year ...
Manufactured by:Optinova based inMariehamn, Åland, FINLAND
Optinfusion™ Standard Inventory is our over-the-counter package of IV Catheter tubing made from FEP and TPU with three encapsulated radiopaque ...
Manufactured by:Delta Med based inViadana, ITALY
Standard intravenous PUR (Polyurethane) and FEP (Teflon) Delta Med catheters are developed and manufactured by highly skilled technicians to ensure safe and quality ...
Manufactured by:Delta Med based inViadana, ITALY
The intravenous safety catheters in PUR (Polyurethane) and FEP (Teflon) Delta Med are developed and manufactured by highly specialized technicians to ensure safe and quality ...
Manufactured by:Sol-Millennium Medical Group based inSuwanee, GEORGIA (US) (USA)
The SOL-VET IV catheter needle features a translucent, colored hub that allows for easy identification of gauge, as well as immediate flashback ...
Manufactured by:Tytek Medical Inc. based inWest Chester Township, OHIO (USA)
TyTek Medical’s 14 and 10 gauge TPAK chest decompression needles are the recommended 3.25" length for treating a tension pneumothorax. You can't count on a standard IV catheter to needle a chest deeply enough in needle thoracostomy. Our 14 and 10 gauge x 3.25" TPAK chest decompression needles are a compact, reliable solution for treating a tension ...
Manufactured by:Attwill Medical Solutions based inLodi, WISCONSIN (USA)
FILTERGuard™ is a disposable IV filter with CHG. The sterile, single-use disposable IV filter is infused with chlorhexidine gluconate (CHG) to help reduce the incidence of bloodstream infections, local infections and skin colonization in patients with IV catheters. This innovative product is designed as an in-line accessory ...
by:Velano Vascular based inSan Francisco, CALIFORNIA (USA)
PIVO is a needle-free, single-use, sterile device that temporarily attaches to a peripheral IV catheter to collect a fresh venous sample. Using the existing PIV line as a conduit to the vein, a flexible, internal flow tube is advanced through the PIV, beyond the catheter tip, and into the vessel to collect a blood sample. This flow tube is ...
Manufactured by:DETECTO - Cardinal Scale Manufacturing Company based inWebb City, MISSOURI (USA)
DETECTO Rescue Series Anesthesiology Medical Cart, 5 Blue ...
Manufactured by:DETECTO - Cardinal Scale Manufacturing Company based inWebb City, MISSOURI (USA)
DETECTO Rescue Series ER Medical Cart, 6 Red ...
Manufactured by:DETECTO - Cardinal Scale Manufacturing Company based inWebb City, MISSOURI (USA)
DETECTO Rescue Series Isolation Medical Cart, 6 Yellow ...
Manufactured by:Applied Medical Technology, Inc. (AMT) based inBrecksville, OHIO (USA)
The CINCH® is a practical solution for keeping medical tubing securely in place. Simply clean the desired skin area, apply the CINCH® to your body, place your medical tubing within the device and secure the tubing with the silicone strap. The CINCH® helps prevent medical tubing from becoming tangled or snagged. Medical tubing doesn’t have to slow you down. We’ve combined a ...
Manufactured by:Flex Ltd based inSan Jose, CALIFORNIA (USA)
Delivering tight tolerances and repeatability. Whether your product is high precision or general use, we can help build your single-use products, such as IV bags, diagnostic catheters, tubing sets and other disposable devices, in high volume. Our manufacturing is automated to help lower costs and improve quality. And our supply chain is vertically integrated, ...
Manufactured by:Martam Medical, LLC based inBoca Raton, FLORIDA (USA)
FlexAgrip Catheter Securement Device is a catheter securement device having a self-adhesive backing and a rigid plastic material with shape memory capabilities to allow stabilization of medical ...
Manufactured by:Lineus Medical based inFayetteville, ARKANSAS (USA)
Harmful forces on peripheral IV lines can lead to dressing disruption, phlebitis, dislodgement, occlusion, infiltration and ultimately to an IV restart. The IV line shown in Figure 1 below has not dislodged, but no one should have a damaging force like this placed on their IV line. SafeBreak Vascular separates, as shown in the video in Figure 2, to remove these harmful forces ...