Refine by
Nasal Aspirate Equipment Supplied In Usa
6 equipment items found
Manufactured by:Microlife Corporation based inWidnau, SWITZERLAND
The Microlife electric nasal aspirator BC 50 is intended to be used for providing intermittent suction to remove nasal secretions and mucous in children from 0+ months old. It gently and quickly removes excess mucus for alleviation of infection of the upper respiratory tract. ...
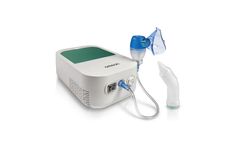
Manufactured by:Omron Healthcare, Inc based inHoofddorp, NETHERLANDS
DuoBaby is a unique 2in1 compressor nebulizer with integrated nasal aspirator that can help treat the most common respiratory conditions of babies. The nasal aspirator gently cleans the baby’s nose, reducing congestion and allowing him to breath freely. The nebulizer enables efficient medication delivery to the upper and ...
Manufactured by:Lumiquick Diagnostics, Inc based inSanta Clara, CALIFORNIA (USA)
Influenza A & B Test is an in vitro immunochromatographic assay for the qualitative detection of influenza type A and type B nucleoprotein antigens in nasopharyngeal swab, nasal swab and nasal aspirate samples. The identification is based on the monoclonal antibodies specific for the nucleoprotein of Influenza virus A and B. ...
Manufactured by:Genabio Diagnostics Inc. based inBedford, MASSACHUSETTS (USA)
cIntended Use: Fosun COVID-19 RT-PCR Detection Kit is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from the SARS-CoV-2 in upper and lower respiratory specimens (such as anterior nasal swabs, mid-turbinate nasal swabs, nasopharyngeal swabs, oropharyngeal swabs, sputum, lower respiratory tract aspirates, ...
Manufactured by:Access Bio based inSomerset, NEW JERSEY (USA)
The CareStart™ COVID-19 MDx RT-PCR is a real-time reverse transcription polymerase chain reaction (RT-PCR) test intended for the qualitative detection of nucleic acid from SARS-CoV-2 in respiratory specimens (such as nasopharyngeal, oropharyngeal and nasal swabs, and nasopharyngeal wash/aspirate or nasal aspirate) and ...
Manufactured by:Hologic, Inc. based inMarlborough, MASSACHUSETTS (USA)
The emergence of SARS-CoV-2 was unforeseen, and the worldwide outbreak of COVID-19 has already impacted millions. In response to the desperate public need for rapid high-volume testing we developed SARS-CoV-2 assays on both our Panther® and Panther Fusion® systems.* Both assays enable labs to run over 1000 tests in 24 hours and attain first results in 3.5 hours or ...