- Home
- Equipment
- azerbaijan
- open heart surgery
Refine by
Open Heart Surgery Equipment & Supplies In In Azerbaijan
16 equipment items found
Manufactured by:Zimmer Biomet CMF and Thoracic based inJacksonville, NEW JERSEY (USA)
An innovative, sternal closure system designed to approximate, compress and rigidly fixate the sternum following open-heart surgery in patients with poor bone ...
Manufactured by:Valcare Medical based inHerzlyia Pituach, ISRAEL
AMEND mitral repair implant is an innovative D-shaped ring with unique anchoring capabilities that emulates the current gold-standard annuloplasty rings used in open-heart surgery. It is delivered into the mitral annulus in a transcatheter transeptal procedure. The implant emerges from the catheter in a linear form and transforms into a closed ...
Manufactured by:P+F Products + Features GMBH based inVienna, AUSTRIA
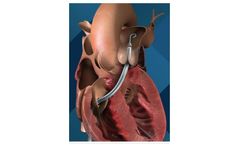
The Vienna Aortic Self-Expandable Transcatheter Valve is designed to treat severe aortic stenosis in patients at medium to high risk for open heart surgery. The minimally invasive heart valve replaces the native valve without the need for concomitant surgical removal and is placed throught femoral access. The new generation ...
Manufactured by:Newmedical Technology Inc. based inNorthbrook, ILLINOIS (USA)
2" x 8" silicone strips fill the need for a strip bigger than our 1" x 6" strips and shorter than our 2" x 24" abdominal strip. Designed for scars 7" or less, including those resulting from mastectomy, open heart surgery, hysterectomy, knee replacement surgery, and spine ...
Manufactured by:Jarvik Heart, Inc. based inNew York, NEW YORK (USA)
A total artificial heart is built to replace both ventricles of the living heart. The entire biological heart (except for the atria) is usually removed, and the man-made device is positioned in its place. Most total artificial hearts mimic the natural heart’s rhythm, drawing and pumping blood in pulses. ...
Manufactured by:Kapp Surgical Instrument Inc. based inCleveland, OHIO (USA)
May be used prophylactically during any cardiac procedure where there is access to the LA appendage for those patients not yet in a-fib to eliminate future need for anticoagulation should a-fib develop. Ease of use – placed in ideal location at the base of the LA appendage during routine open heart surgery without adding significantly to ...
Manufactured by:APC Cardiovascular Ltd. based inHartford, UNITED KINGDOM
Myo-Pace heart wires are a range of myocardial heart wires designed specifically for use as a temporary cardiac pacing wire after open heart surgery. The polyethylene insulation provides the smooth surface necessary to minimise tissue trauma both on placing and withdrawal of the heart wire, ...
Manufactured by:Abiomed based inDanvers, MASSACHUSETTS (USA)
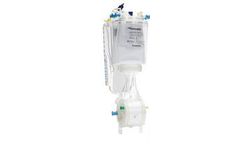
The Impella RP heart pump provides temporary, circulatory support for patients who develop right heart failure. It is the only FDA-approved heart pump indicated for patients experiencing acute right heart failure or decompensation following left ventricular assist device implantation, myocardial infraction, heart ...
Manufactured by:Relisys Medical Devices Limited based inRanga Reddy, INDIA
The VIENNA AORTIC VALVE SE self-expandable transcatheter valve is designed to treat severe Aortic stenosis in patients at medium to high risk for open heart surgery. The minimal invasive heart valve replaces the native valve without the need for concomitant surgical removal and is placed via the femoral access. The new generation ...
Manufactured by:Springboard based inRancho Cordova, CALIFORNIA (USA)
Surgical Device Component Manufacturer; Although Springboard does not make any finished medical devices, we are an industry-leading surgical device component manufacturer. We supply a sizeable number of injection-molded surgical components that go into life-saving surgical devices. At Stack, we understand that our parts are used in devices that can save lives, and that the failure of insert ...
Manufactured by:Terumo based inShibuya-ku, JAPAN
Terumo offers devices that circulate blood in place of the heart and lungs during cardiovascular surgery in which the heart is stopped (“on pump” ...
Manufactured by:Sterlitech Corporation based inAuburn, WASHINGTON (USA)
Sterlitech Polyethersulfone (PES) membrane is a hydrophilic membrane constructed from pure polyethersulfone polymer membrane. PES membrane filters are designed to remove particulates during general filtration. Their low protein and drug binding characteristics also make polyethersulfone (PES) membrane disc filters ideally suited for use in life science ...
Manufactured by:Best Hope Precision Device Co., Ltd based inJiaxing City, CHINA
Aortic stenosis is a common disease where one of the heart valves narrows, restricting the flow of blood into the body. When it gets bad enough, the diseased valve often needs to be ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
The Cardioblate Surgical Ablation Pen is intended to ablate cardiac tissue during cardiac surgery using radiofrequency ...
Manufactured by:Dogsan based inTrabzon, TURKEY
DOPACE Temporary Pacing Wire is twisted 316L stainless steel wire, with polyethylene coated (sheathed), with double needle, with or without anchor. DOPACE meets requirements established by the European Pharmacopoeia (EP) and United States Pharmacopoeia (USP) for non-absorbable surgical sutures, except insulation thickness. DOPACE may also be labeled with the B&S gauge ...
Manufactured by:Destiny Pharma plc based inBrighton, UNITED KINGDOM
A Phase 2b clinical study, which was a multi-centre, randomized, placebo-controlled study of XF-73 nasal gel as a new product for the prevention of the incidence of post-surgical infections such as methicillin-resistant Staphylococcus aureus (MRSA) completed recruitment at the end of 2020 and top-line results were reported in Q1 2021, as ...