- Home
- Equipment
- australasia
- osteoconductivity
Refine by
Osteoconductivity Equipment Supplied In Australasia
9 equipment items found
Manufactured by:Xtant Medical based inBelgrade, MONTANA (USA)
The Atrix-C Union allograft cervical interbody spacer provides an osteoconductive matrix creating an optimal environment for bone growth and fusion. Atrix-C Union allografts are available in three footprints and eight heights to accommodate a wide range of patient anatomy while providing a steady platform for ...
Manufactured by:TriMed Inc. based inSanta Clarita, CAMBODIA
A synthetic, osteoconductive and bioactive material. Manufactured by Bonalive Biomaterials Ltd in Finland from a unique S53P4 bioactive glass, characterized by its ability to firmly attach to living tissue. Indicated for bony voids or gaps that are not intrinsic to the stability of the bony ...
by:In2Bones Global based inMemphis, TENNESSEE (USA)
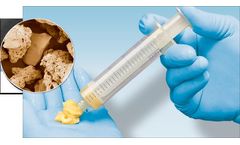
Consisting of hydroxyapatite, tricalcium phosphate and bioactive glass in a collagen matrix, the Backfill Plugs are sized to easily fit in surgically created osseous defects; 5.5mm dia. (for 6mm void) and 7.5mm dia. (for 8mm void) by 40mm in length. The osteoconductive scaffold rapidly absorbs the surrounding blood and fluids to aid in new bone formation during the healing ...
Manufactured by:Xtant Medical based inBelgrade, MONTANA (USA)
Derived from 100% human bone – maximum allograft content; no extraneous carriers that may impede bone healing; Osteoinductive potential – validated for positive osteoinductive capacity; Osteoconductive – DBM provides an ideal scaffold that directs and supports bone formation; Excellent handling characteristics – retains shape and resists irrigation and ...
Manufactured by:Xtant Medical based inBelgrade, MONTANA (USA)
Osteoinductive Potential- Cortical fiber demineralized bone matrix provides a high quality osteoinductive signal. Additional growth factors are captured from bone lining cells and restored back into the product.* Osteoconductive- The cortical fiber demineralized bone matrix and cancellous bone creates a robust scaffold. Ease of Use- 10 minute thaw time in a room temperature water ...
Manufactured by:Xtant Medical based inBelgrade, MONTANA (USA)
Matriform Si was developed to resemble the composition and porous structure of natural human bone. Comprised of 96% pure phase β-TCP granules, 4% silicate and collagen, Matriform Si provides the ideal biomimetic scaffold for spinal fusion procedures. The flexible strip offers excellent handling and shape memory ensuring direct contact with the surface of healthy ...
Manufactured by:Xtant Medical based inBelgrade, MONTANA (USA)
Xtant’s 3Demin Technology utilizes demineralized cortical bone fibers that are entangled and shaped into sizes engineered to complement specific surgical applications. This unique process creates an interconnected graft material that contains BMPs and other growth factors necessary for the promotion of new bone formation. 3Demin allografts are flexible upon hydration and each allograft has ...
by:In2Bones Global based inMemphis, TENNESSEE (USA)
Synergistic Combination of Bioactive Glass and Demineralized Bone Matrix. Bioactive Matrix is a novel bone graft substitute expressly designed to optimize surgical handling, graft stability and osteoproductivity. ...
by:Bonalive Biomaterials Ltd based inTurku, FINLAND
Bonalive putty is a highly moldable, easy-to-apply bone regeneration technology that naturally stimulates bone ...