- Home
- Equipment
- asia middle east
- point of care poc multi measurements testing
Show results for
Refine by
Locations
- Asia & Middle East
- Afghanistan
- Armenia
- Azerbaijan
- Bahrain
- Bangladesh
- Bhutan
- Brunei Darussalam
- Cambodia
- China
- East Timor
- Georgia
- Guam
- India
- Indonesia
- Iran
- Iraq
- Israel
- Japan
- Jordan
- Kazakhstan
- Kuwait
- Kyrgyzstan
- Laos
- Lebanon
- Malaysia
- Maldives
- Mongolia
- Myanmar
- Nepal
- North Korea
- Oman
- Pakistan
- Palestinian Territories
- Philippines
- Qatar
- Russia
- Saudi Arabia
- Singapore
- South Korea
- Sri Lanka
- Syria
- Taiwan
- Tajikistan
- Thailand
- Turkmenistan
- United Arab Emirates
- Uzbekistan
- Vietnam
- Yemen
Point Of Care Poc Multi Measurements Testing Equipment & Supplies In In Asia Middle East
101 equipment items found
by:Tellgen Corporation based inShanghai, CHINA
Throughput: 1 test per 30 minutes. Heating: 61 °C. Linearity: r>0.99. Channels: FAM/ROX. Connection: Bluetooth/USB. Power: 100-240V AC 50/60Hz. Size: 132*102.5*85mm. Weight: ...
by:Tellgen Corporation based inShanghai, CHINA
Throughput: 1 test per 30 minutes. Heating: 61 °C. Linearity: r>0.99. Channels: FAM/ROX. Connection: Bluetooth/USB2.0. Power: DC15V 250W. Size: 300*267*198mm. Weight: ...
Manufactured by:Chembio Diagnostic Systems, Inc. based inHauppauge, NEW YORK (USA)
The DPP® Micro Reader is a handheld device that enables testing for SARS-CoV-2 and other DPP® tests. Our reader streamlines the point-of-care testing experience with precise and accurate ...
Manufactured by:BYTEC Medizintechnik GmbH based inEschweiler, GERMANY
Detection of animal diseases; Mobile (analysis) platform; Gaining genetic information; Process is based on disposable cartridge (all chemicals included); Mobile app connection; Very ...
Manufactured by:Owen Mumford Ltd. based inOxfordshire, UNITED KINGDOM
Contact activation - Designed for seamless sampling. Unistik® Touch is designed to make capillary sampling as simple and seamless as possible. With one-touch activation, you simply press the device against the sample site, allowing it to activate automatically under pressure. Once activated, the needle retracts into the body of the device, minimizing the risk of re-use, cross-infection and ...
Manufactured by:Chembio Diagnostic Systems, Inc. based inHauppauge, NEW YORK (USA)
The DPP® Micro Reader II is our newest, CE Marked, handheld device that enables testing for SARS-CoV-2. Our reader streamlines the point-of-care testing experience with precise and accurate ...
Manufactured by:Biomerica, Inc. based inIrvine, CALIFORNIA (USA)
Available as single Test Strip, single Test Device, or Multi Panel (2-12 on one ...
Manufactured by:Biomerica, Inc. based inIrvine, CALIFORNIA (USA)
Available as single Test Strip, single Test Device, or Multi Panel (2-12 on one ...
Manufactured by:Biomerica, Inc. based inIrvine, CALIFORNIA (USA)
Available as single Test Strip, single Test Device, or Multi Panel (2-12 on one ...
Manufactured by:Biomerica, Inc. based inIrvine, CALIFORNIA (USA)
Available as single Test Strip, single Test Device, or Multi Panel (2-12 on one ...
Manufactured by:Sekisui Diagnostics, LLC based inBurlington, MASSACHUSETTS (USA)
Detects elevated vaginal fluid sialidase activity, an enzyme produced by bacterial pathogens associated with bacterial vaginosis including Gardnerella, Bacteroides, Prevotella and ...
by:Diabetomics, Inc. based inHillsboro, OREGON (USA)
The Lumella® Test, The New Point-of-Care Blood Test Helps in Early Detection of Preeclampsia; The Lumella® test estimates the concentration of Glycosylated Fibronectin, a new pregnancy specific biomarker to accurately assess the risk of preeclampsia. The Lumella® test requires a simple finger stick and can be performed in physician’s office/clinic in the first, second, or third ...
Manufactured by:Chembio Diagnostic Systems, Inc. based inHauppauge, NEW YORK (USA)
A CE Marked rapid point-of-care test that simultaneously and separately detects treponemal and nontreponemal ...
by:Diabetomics, Inc. based inHillsboro, OREGON (USA)
The first and only point-of-care tests for early detection of type-1 diabetes and LADA (Latent Autoimmune Diabetes in ...
Manufactured by:Sekisui Diagnostics, LLC based inBurlington, MASSACHUSETTS (USA)
With the Acucy® Influenza A&B Test, clinicians can test and provide fast and accurate results that aid in the treatment of a patient in one office visit, enhancing the patient experience, reducing the spread of infection and improving patient ...
Manufactured by:Atomo Diagnostics Limited based inLeichhardt, AUSTRALIA
The AtomoRapid Franklin RDT platform offers all-in-one convenience with a built-in safety lancet, innovative blood collection feature, integrated blood and buffer delivery system, and digital LCD result ...
by:Diabetomics, Inc. based inHillsboro, OREGON (USA)
First rapid point-of care test for detection of rheumatoid arthritis; Non-invasive oral fluid or finger stick sample; Results in 15 minutes; ACA rapid, point-of-care test for assessment of anti-citrullinated human serum albumin (HSA) autoantibodies (ACAs) and Anti-citrullinated peptides auto antibodies (ACCP)* for screening and diagnosis of RA and potential companion diagnostic role with ...
Manufactured by:DxGen Corp. based inGunpo-si, SOUTH KOREA
Semi-Automatic Analyzer for Point-Of-Care Testing measuring HbA1c, Glycated Albumin, CRP, Hemoglobin, COVID-19 Antibody (IgM/IgG), and Antigen. ...
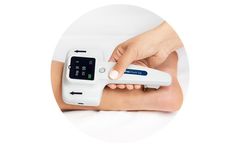
Manufactured by:NeuroMetrix, Inc. based inWoburn, MASSACHUSETTS (USA)
An accurate and fast point-of-care test for detecting a common form of nerve degeneration called peripheral neuropathy. DPNCheck is a fast, accurate, and quantitative nerve conduction test that is used to evaluate nerves for evidence of peripheral neuropathy, such as diabetic peripheral neuropathy (DPN). It is designed to be used by clinicians at the point-of-care to objectively detect, stage, ...
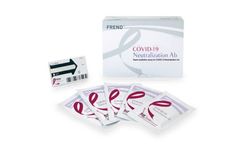
Manufactured by:NanoEnTek, Inc. based inGuro-gu, SOUTH KOREA
The FREND COVID-19 Neutralization Ab is a point-of-care testing (POCT) which can be used to check whether a patient has developed the immune response to SARS-CoV-2 using human serum or plasma ...