Refine by
Preclinical Data Equipment Supplied In Europe
19 equipment items found
Manufactured by:Adaptimmune LLC based inPhiladelphia, PENNSYLVANIA (USA)
What differentiates this therapy from our ADP-A2M4 SPEAR T-cells is that these cells also express the CD8α co-receptor alongside the engineered TCR that targets MAGE-A4. Preclinical data indicate that co-expression of CD8α may broaden the immune response against solid tumors and increase anti-tumor activity by leveraging CD4+ cells into CD8+ killer or ...
Manufactured by:Biosceptre International Limited based inCambridge, UNITED KINGDOM
We are also developing antibodies that specifically target nfP2X7 for use in both solid and haematological tumours. Our preclinical data is very promising and studies show an excellent safety profile. By exploiting the modular nature of the antibody structure, the same panel of antibody sequences can be used to generate a variety of therapeutic antibody formats ...
Manufactured by:Brenus Pharma based inIssoire, FRANCE
An allogeneic first in class immunotherapy harnessing the patient immune system to fight immune tolerance and prevent cancer ...
by:Redbiotec AG based inSchlieren, SWITZERLAND
Redbiotec specializes in developing high-quality vaccines to fight difficult diseases. After successfully spinning out the CMV vaccine program that was acquired by Pfizer, Redbiotec is currently developing HSV-2 therapeutic vaccine ...
by:Hookipa Pharma Inc. based inVienna, AUSTRIA
Our scientific platform includes two distinct approaches to arenavirus-based therapies: replicating and non-replicating. Our replicating technology is designed to create a stronger immune response that we believe is more appropriate for use in oncology. Our non-replicating technology is designed to evoke a potent immune response for prophylactic use against infectious disease. ...
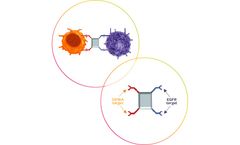
Manufactured by:Affimed GmbH based inHeidelberg, GERMANY
Lighting the way with clinical results: we are creating success with monotherapies and combination therapies. AFM13, a first-in-class ICE (innate cell engager) molecule targeting patients with CD30-positive lymphomas,4 showed antitumor responses in a Phase 1/2 trial as a monotherapy in 15 patients with T-cell lymphomas and cutaneous involvement (44% ORR).5 When used in combination with allogeneic ...
Manufactured by:Sequana Medical NV based inSint-Denijs Westrem, BELGIUM
Direct Sodium Removal (DSR®) therapy is Sequana Medical’s novel and proprietary approach under development for the management of fluid overload due to heart failure. The sodium concentration in patients with fluid overload is in balance but there is too much sodium and too much fluid in the body. Sequana Medical’s approach is to remove excess sodium in patients with ...
Manufactured by:Applied Pharma Research s.a. based inBalerna, SWITZERLAND
APR TD012 is a clinical stage drug candidate for the treatment of wounds in Hailey Hailey Disease (HHD) patients. The product improves healing process thanks to its bioburden control and its anti-oxidant properties.Oxidative stress has been demonstrated to play a specific role in the pathogenesis of HHD by regulating the expression of factors playing an important role in keratinocyte ...
Manufactured by:Affimed GmbH based inHeidelberg, GERMANY
AFM24, an EGFR-directed ICE® (innate cell engager) molecule, represents a distinctive mechanism that engages innate immune cells by recruiting NK cells and macrophages to the site of the tumor for effective and efficient tumor-cell killing. Potential to treat various solid tumor types with a more acceptable safety profile while remaining immune to the challenge of ...
by:Abivax based inParis, FRANCE
ABX196 has been developed as a synthetic agonist of iNKT cells from a proprietary platform technology that identifies cells with immune enhancing potential in cancer ...
by:Procarta Biosystems Ltd based inNorwich, UNITED KINGDOM
Proprietary nanoparticles deliver an oligonucleotide inside the bacterial pathogen. The oligonucleotide acts as a Transcription Factor Decoy (TFD): it contains the binding site of a transcription factor that controls a large set of genes required for the survival of the bacterium. The TFD acts as a competitive inhibitor to prevent these genes being turned on and so prevents growth and treats the ...
by:Abivax based inParis, FRANCE
ABX464 is an oral, first-in-class, small molecule that has demonstrated safety and profound anti-inflammatory activity in the completed Phase 2a and Phase 2b induction studies as well as in long-term maintenance studies in patients with moderate-to-severe ulcerative colitis (UC). In addition to the clinical observations in UC patients, Abivax generated excellent phase 2a clinical results in RA, ...
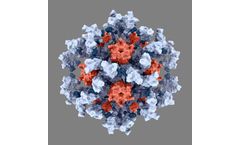
Manufactured by:AstraZeneca based inCambridge, UNITED KINGDOM
IVX-411 targets SARS-CoV-2, the coronavirus that causes COVID-19. Developed using cutting-edge structure-based design techniques at the Institute for Protein Design at the UW School of Medicine, IVX-411, our lead vaccine candidate for COVID-19, is a self-assembling protein nanoparticle that displays 60 copies of the SARS-CoV-2 spike (S) glycoprotein receptor-binding domain (RBD) in a highly ...
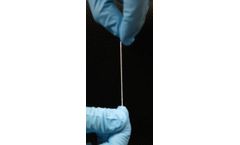
Manufactured by:Fibralign Corporation based inUnion City, CALIFORNIA (USA)
The BioBridge® Collagen Matrix is an FDA cleared surgical mesh designed for use in soft tissue support and repair. This thread-like device is made of medical-grade collagen, fabricated using Fibralign’s patented Nanoweave® technology. This novel product is the result of years of significant research collaboration with Stanford University and UCSF to optimize its nanostructure and ...
Manufactured by:OSE Immunotherapeutics based inNantes, FRANCE
OSE-230 is an agonist antibody against ChemR23, also known as chemerin chemokine-like receptor 1 (CMKLR1), a G-protein coupled receptor (GPCR) expressed on myeloid immune cells known to modulate inflammation. Persistent inflammation is a characteristic feature of all chronic inflammatory or autoimmune diseases and if not controlled or resolved, it can lead to further tissue damage and give rise ...
by:CDR-Life based inSchlieren, SWITZERLAND
CDR-Life’s T cell engaging antibodies are potentially transformative in the field of cancer immunotherapies» Prof. Markus G. Manz, MD, Head of Medical Oncology and Hematology at University Hospital and University of Zurich, Switzerland. CDR-Life’s pipeline is based on it’s unique antibody fragment platform. It includes a series of highly cancer-targeted and drug-based ...
Manufactured by:Biomed Lublin S.A. based inLublin, POLAND
Name of the Medicinal Product: GAMMA anty-HBs 200, 200 IU/ml solution for injection. Qualitative And Quantitative Composition: Human hepatitis B immunoglobulin. 1 ml of the solution contains human protein not less than 100 mg, of which at least 85% is immunoglobulin G (IgG) with anti-HBs antibody content 200 IU. The product is manufactured from human donor serum. For the full list of excipients, ...
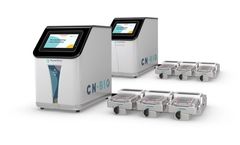
Manufactured by:CN Bio based inCambridge, UNITED KINGDOM
Generate human-specific pre-clinical safety and efficacy data with pioneering PhysioMimix single- and multi-organ-on-a-chip ...
Manufactured by:Biomed Lublin S.A. based inLublin, POLAND
Name Of The Medicinal Product; DISTREPTAZA; Streptokinase + Streptodornase; 15000 IU + 1250 IU. Rectal ...