Show results for
Refine by
Real Time Microchip Based Pcr Equipment Supplied In Italy
29 equipment items found
Manufactured by:Dia.Pro Diagnostic Bioprobes s.r.l based inSesto San Giovanni, ITALY
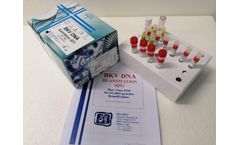
The BKV DNA Quantitation Real-Time PCR kitcoded BKVDNAQT.CE is intended for the quantitative detection of the BK Virus DNA in human plasma and urine with a simultaneous control of the amplification/extraction reaction through an Internal Control (IC). The kit has been adapted for the use on the Real-Time Thermacyclers ABI 7500 Sequence Detection System® (Software SDS version 1.3.1, ...
Manufactured by:Dia.Pro Diagnostic Bioprobes s.r.l based inSesto San Giovanni, ITALY
The Enterovirus RNA Real-Time PCR kit coded ENTERORNA.CE is intended for the qualitative detection of Enterovirus RNA in human plasma and CSF samples (Cerebral Spinal Fluid) with a simultaneous control of the amplification reaction through an Internal Control (IC). ...
Manufactured by:Dia.Pro Diagnostic Bioprobes s.r.l based inSesto San Giovanni, ITALY
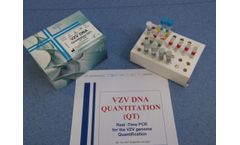
The VZV DNA Quantitation (QT) Real-Time PCR kit coded VZVDNAQT.CE is intended for the quantitative detection of Varicella-Zoster virus DNA in human plasma and CSF (cerebrospinal fluid) with a simultaneous control of the extraction/amplification reaction through an Internal Control (IC). ...
Manufactured by:AB ANALITICA s.r.l. based inPADUA, ITALY
The REALQUALITY RQ-CMV STANDARD is a CE-IVD diagnostic kit containing known quantified standards for the quantification of Cytomegalovirus (CMV) by Real Time PCR. This product has to be used in combination with the REALQUALITY RQ-CMV kit. ...
Manufactured by:Sentinel CH. SpA based inMilano, ITALY
One Step Multiplex Real-Time RT-PCR mix. All targets in one reaction. STAT-NAT is Sentinel Diagnostics’ proprietary technology developed with a new protective compound able to stabilize the activity of enzymes for a very long time with no temperature controlled storage requirements. STAT-NAT kits contain all reagents and buffers required to perform the reaction. Lyophilized reagents are ...
Manufactured by:Dia.Pro Diagnostic Bioprobes s.r.l based inSesto San Giovanni, ITALY
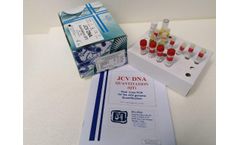
The JCV DNA Quantitation Real-Time PCR kit coded JCVDNAQT.CE is intended for the quantitative detection of the JC Virus DNA in human plasma ,urine and cerebrospinal fluid (CSF) with a simultaneous control of the amplification/extraction reaction through an Internal Control (IC). ...
Manufactured by:AB ANALITICA s.r.l. based inPADUA, ITALY
Kit for the detection of Hepatitis C virus (HCV) genotypes 1 to 7 and subtypes a and b of genotype 1 by reverse transcription, PCR and Reverse Line Blot of the 5’UTR and CORE region. The kit can accurately detect subtypes a and b of genotype 1, thanks to the use of dual targets: 5’UTR and CORE. The reverse transcription and amplification (One-Step RT-PCR) mix contains the dUTP/UNG ...
Manufactured by:Eurospital Diagnostic Division | Eurospital Spa based inTrieste, ITALY
Molecular biology kit for the determination of susceptibility to celiac disease by Real Time PCR amplification of human DNA from peripheral blood and further identification of specific alleles for this condition. Genotype, Risk, Prevention. The most innovative feature of XeliGen is the identification of subjects who are at risk of developing coeliac disease. By using XeliGen is possible to detect ...
Manufactured by:Generon S.p.A based inSan Prospero (MO), ITALY
SPECIALfinder SpyX 1000 ppm is designed for the process control using Real-Time PCR or immunochemical approach or for the quantitative analysis of different allergens in food samples using Real-Time PCR approach. This reference material contains the target allergen in a proven homogenous concentration of 1000 ppm dispersed in a corn flour ...
Manufactured by:Sentinel CH. SpA based inMilano, ITALY
3-gene based Real Time RT-PCR for qualitative detection of SARS-CoV-2. STAT-NAT is Sentinel Diagnostics’ proprietary technology developed with a new protective compound able to stabilize the activity of enzymes for a very long time with no temperature controlled storage requirements. STAT-NAT kits contain all reagents and buffers required to perform the reaction. Lyophilized reagents are ...
Manufactured by:Diatech Pharmacogenetics srl based inJesi (AN), ITALY
Main features Detection of the main mutations of codon 600 of the gene BRAF using 5 oligo mixes. Each mix allows the co-amplification of one or more mutated alleles plus an endogenous control gene. A specific oligo control mix enables the evaluation of the quality and the quantity of the DNA in each sample. Control DNA positive for all the mutations detected by the ...
Manufactured by:Diatech Pharmacogenetics srl based inJesi (AN), ITALY
Main features Detection of the main mutations of exons 18, 19, 20, 21 of EGFR gene using 8 oligo mixes. Each mix allows the co-amplification of one or more mutated alleles plus an endogenous control gene. A specific oligo control mix enables the evaluation of the quality and the quantity of the DNA in each ...
Manufactured by:Diatech Pharmacogenetics srl based inJesi (AN), ITALY
Main features Detection of the main mutations of exon 2 (codons 12, 13), of exon 3 (codons 59, 61) and of exon 4 (codons 117, 146) of the gene KRAS using 12 oligo mixes. Each mix allows the co-amplification of one or more mutated alleles plus an endogenous control gene. A specifc oligo control mix enables the evaluation of the quality and the quantity of the DNA in each ...
Manufactured by:Diatech Pharmacogenetics srl based inJesi (AN), ITALY
Detection of the main mutations of exon 2 (codons 12, 13), of exon 3 (codons 59, 61) and of exon 4 (codons 117, 146) of the gene KRAS using 8; oligo mixes. Each mix allows the co-amplification of one or more mutated alleles plus an endogenous control gene. A specific oligo control mix enables; the evaluation of the quality and the quantity of the DNA in each ...
Manufactured by:Diatech Pharmacogenetics srl based inJesi (AN), ITALY
Detection of the main mutations of codon 600 of the gene BRAF using 4 oligo mixes. Each mix allows the co-amplification of one or more mutated alleles plus an endogenous control gene. A specific oligo control mix enables the evaluation of the quality and the quantity of the DNA in each ...
Manufactured by:Sentinel CH. SpA based inMilano, ITALY
Real Time RT-PCR for qualitative detection of novel Coronavirus SARS-CoV-2, FLU A&B and RSV. STAT-NAT® is Sentinel Diagnostics’ proprietary technology developed with a new protective compound able to stabilize the activity of enzymes for a very long time with no temperature controlled storage requirements. STAT-NAT kits contain all reagents and buffers required to perform the ...
by:Diasorin based inSALUGGIA, ITALY
Sample-to-answer, real-time PCR systems designed for improved laboratory efficiency and workflow. ARIES and ARIES M1 Systems are Luminex’s sample-to-answer, real-time PCR systems that are crafted to increase laboratory efficiency, ensure result accuracy, and fit seamlessly into today’s lean ...
Manufactured by:CLONIT srl based inAbbiategrasso, ITALY
The AML1-ETO t(8;21)(q22;q22) is an in vitro diagnostic test for the identification and quantification of the AML1-ETO gene fusion transcripts resulting from t(8;21)(q22;q22) , by one-step Real-Time PCR. The AML1-ETO t(8;21)(q22;q22) assay allows also the detection and quantification of ABL as a endogenous control gene. Standard curves of known amounts of both the endogenous control and the gene ...
Manufactured by:CLONIT srl based inAbbiategrasso, ITALY
The PML-RARA t(15;17)(q22;q21) is an in vitro diagnostic test for the detection and identification of the PML-RARA gene fusions resulting from the t(15;17)(q22;q21) , by multiplex one-step Real-Time PCR. The PML-RARA t(15;17)(q22;q21) allows also the detection and quantification of ABL as a endogenous control gene. Standard curves of known amounts of both the endogenous control and the gene ...
Manufactured by:Diatheva Srl based inCartoceto, ITALY
Coronaviruses are a large family of viruses that are common in people and many different species of animals including camels, cattle, cats, and bats. In December 2019, a cluster of patients with a novel coronavirus was identified in Wuhan, China. The virus named SARS-CoV-2 can cause the disease named coronavirus disease 2019 (COVID-2019) [WHO, 2 March 2020]. PCR testing of asymptomatic or ...