Refine by
Respiratory Infection Equipment Supplied In Usa
56 equipment items found
Manufactured by:Microbion Corporation based inBozeman, MONTANA (USA)
Cystic Fibrosis (CF) lung disease, which is estimated to affect more than 30,000 in the US and 70,000 worldwide1, is characterized by chronic bacterial infection and severe inflammation that leads to progressive deterioration in lung function. Chronic pulmonary infections in CF patients, a significant portion which involves Pseudomonas aeruginosa, are characterized by persistence, biofilm ...
Manufactured by:SpeeDx based inSydney, AUSTRALIA
Detect COVID-19 causative coronavirus, SARS-CoV-2; Multiplex RT-qPCR test with dual targets (RdRp/ORF1ab) and RNA-based internal control in a single-well. Scalable solution supporting surge ...
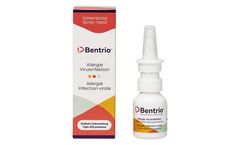
Manufactured by:Auris Medical AG based inBasel, SWITZERLAND
This thin film is designed to prevent the contact of viruses or allergens with cells; in addition, the composition serves to bind such particles and help with their discharge and to humidify the nasal mucosa. Together, this is designed to reduce the risk of upper respiratory tract viral infections and promote alleviation of allergic ...
Premium
Manufactured by:Lumex Instruments based inMission, BRITISH COLUMBIA (CANADA)
COVID-19 caused by the SARS-COV-2 virus, and influenza by influenza viruses are respiratory tract infections that display overlapping symptoms. Moreover, novel strains of influenza A and B viruses are unpredictable and may cause pandemics like 1918, 1957, 1968, 1977, 2009 by an H1N1, H2N2, H3N2, H1N1, and H1N1/09 subtypes, respectively. ...
Manufactured by:Lumos Diagnostics based inSarasota, FLORIDA (USA)
FebriDx is a rapid, all-in-one point-of-care test device that can differentiate a viral from bacterial acute respiratory infection. FebriDx® can be used to help triage patients at the point of care to reduce uncertainty and avoid unnecessary antibiotics. Results within 10 minutes increases confidence in whether or not to prescribe an antibiotic. ...
Manufactured by:bioMérieux S.A. based inMarcy l`Etoile, FRANCE
Adenovirus R-gene US Assay is a Real Time PCR in vitro diagnostic test for the rapid and qualitative detection of Adenovirus viral DNA isolated and purified from nasopharyngeal swab or nasopharyngeal wash/aspirate specimens obtained from individuals exhibiting signs and symptoms of acute respiratory ...
Manufactured by:N8 Medical, LLC based inPark City, UTAH (USA)
N8 Medical’s initial product will be a ceragenin-eluting CeraShield™ endotracheal tube designed to address a myriad of complications in mechanically-ventilated patients. These patients often develop respiratory infections, which can lead to sepsis and multi-organ failure. ...
Manufactured by:Advansta Inc. based inSan Jose, CALIFORNIA (USA)
In December of 2019, the SARS-CoV-2 virus, which causes the disease COVID-19, was first detected in Wuhan, China. The SARS-CoV-2 virus is a single-stranded RNA coronavirus that causes respiratory infections. The rapid spread of COVID-19 has resulted in a global pandemic which has caused an unprecedented social and economic disruption to daily ...
Manufactured by:Microbion Corporation based inBozeman, MONTANA (USA)
Microbion has two Phase 2 clinical programs in diabetic foot ulcer infection and orthopedic implant infection. In addition, preclinical programs are under development for multiple therapeutic indications, including the treatment of serious respiratory ...
Manufactured by:BiomX based inNess Ziona, ISRAEL
We are developing BX004, a phage therapy for CF patients with chronic Pseudomonas aeruginosa (P. aeruginosa) respiratory infections, a main contributor to morbidity and mortality in this disease. Phase 1b/2a clinical study in CF patients is composed of two parts. Results from Part 1 aimed to evaluate the safety, pharmacokinetics, and microbiologic/clinical ...
Manufactured by:Bio Fire Diagnostics based inSalt Lake City, UTAH (USA)
SARS-CoV-2 is a top concern for patients and clinicians, but several respiratory pathogens can cause nearly indistinguishable symptoms. The FDA De Novo authorized BioFire RP2.1 Panel is a frontline test to help clinicians quickly diagnose respiratory infections, including COVID-19, influenza, RSV, and many ...
Manufactured by:Quidel Corporation based inSan Diego, CALIFORNIA (USA)
Bordetella is a family of small gram-negative bacterial pathogens that cause respiratory tract infections in humans and animals.1 Nine species of Bordetella have been identified to date, but Bordetella pertussis causes most of the human disease and only three additional members, B. bronchiseptica, B. parapertussis, and B. holmesii have been associated with ...
Manufactured by:AstraZeneca based inCambridge, UNITED KINGDOM
IVX-A12 is being designed to target both RSV and hMPV in a single vaccine candidate. hMPV is being increasingly recognized as a major contributor to acute respiratory infection and pneumonia with rates of pneumonia (Clinics in Chest Medicine 2017) and hospitalization (JID 2012) similar to that of RSV and influenza. RSV, hMPV, and influenza seasons show high ...
Manufactured by:Azure Biotech Inc. based inHouston, TEXAS (USA)
Strep A. Specimen Used: Swab/ nasal wash. Test Format: Device. CE mark: ...
Manufactured by:Access Bio based inSomerset, NEW JERSEY (USA)
Influenza is a highly contagious viral infection of the nose, throat, and lungs that occurs most often in the late fall, winter, and early spring. Since other viruses infecting respiratory track have similar symptoms, it is critical to properly diagnose influenza viral infection before any treatment. ...
Manufactured by:TytoCare based inNew York, NEW YORK (USA)
Empowering remote physical exams from anywhere. Tyto device with exam camera and thermometer. Tyto otoscope, stethoscope, and tongue depressor adaptors. Mobile app and clinician dashboard for conducting remote physical exams, reviewing exam data, and communicating with patients. AI-powered guidance technology ensures anyone can capture exam data safely and accurately. Optional backpack available ...
Manufactured by:TytoCare based inNew York, NEW YORK (USA)
Empowering remote physical exams from home: Tyto device with exam camera and thermometer. Tyto otoscope, stethoscope, and tongue depressor adaptors. Mobile app and clinician dashboard for conducting remote physical exams, reviewing exam data, and communicating with patients. AI-powered guidance technology ensures anyone can capture exam data safely and accurately. HIPAA-secure, AWS cloud ...
Manufactured by:Neogen Corporation based inLansing, MICHIGAN (USA)
UNIPRIM is an FDA approved, fast-acting, powerful antibiotic for horses used to treat a wide spectrum of bacterial infections. A combination of 67 mg trimethoprim and 333 mg sulfadiazine per gram provides effective antibacterial activity. UNIPRIM was designed specifically for horses and is cost-effective when compared to other ...
Manufactured by:Omron Healthcare, Inc based inHoofddorp, NETHERLANDS
DuoBaby is a unique 2in1 compressor nebulizer with integrated nasal aspirator that can help treat the most common respiratory conditions of babies. The nasal aspirator gently cleans the baby’s nose, reducing congestion and allowing him to breath freely. The nebulizer enables efficient medication delivery to the upper and lower ...
Manufactured by:Gold Standard Diagnostics based inDavis, CALIFORNIA (USA)
CE marked, rapid and reliable RT-PCR assay for the differentiation of Omicron from other VOCs (Beta, Gamma, and Delta). GSD NovaType IV SARS-CoV-2 specifically identifies S gene mutations K417N, E484K, and L452R. Screening for S gene mutations K417N and L452R allows for active surveillance of variants Omicron and Delta, ...