- Home
- Equipment
- north america
- spine stabilization
Refine by
Spine Stabilization Equipment Supplied In North America
14 equipment items found
Manufactured by:ZimVie Inc. based inPalm Beach Gardens,, FLORIDA (USA)
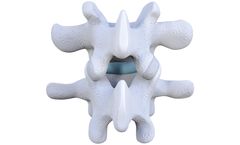
Designed For Success: The MaxAn Anterior Cervical Plate System provides a simple, efficient and innovative approach to anterior cervical plating. The system offers a decompression-based technique for cervical spine stabilization and introduces an innovative, one-level plate technique that provides a direct relationship between the bone graft/spacer size and the ...
Manufactured by:AxioBionics based inAnn Arbor, MISSISSIPPI (USA)
Its modular design allows customization to cater to specific pain areas and medical needs, incorporating lumbar support for enhanced spine stability and abdominal muscle stimulation for core strengthening. The BioBelt's dual-mode electrical stimulation mimics a soothing massage, helping re-educate muscles and support mechanical stability without ...
Manufactured by:Spinal Elements, Inc. based inCarlsbad, CALIFORNIA (USA)
Minimally Invasive Approach: Designed to be performed through a single, small incision. Utilizes a guidewire to accurately place the flexible MicroBlade Shaver® at the point of the decompression ...
Manufactured by:Evolution Spine based inDallas, TEXAS (USA)
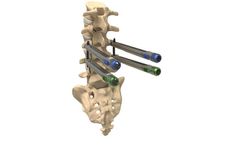
A traditional Pedicle screw system intended to provide immobilization and stabilization of spinal segments in the thoracolumbar spine. Stowe features three tulips (Low, Reduction, MIS). This system allows for manipulation and deformity correction. ...
Manufactured by:Zentech, Inc. based inClearwater, FLORIDA (USA)
The Zenfuze System is a minimally invasive solution for patients suffering from low back pain. Following conservative treatment methods, Zenfuze may be an option. The Zenfuze System can treat conditions such as spinal stenosis, spinal instability, and degeneration. Zenfuze may provide patients with a permanent solution for the conditions associated with low back pain. The majority of patients ...
Manufactured by:ChoiceSpine LLC based inKnoxville, TENNESSEE (USA)
The ChoiceSpine THUNDERBOLT™ Minimally Invasive Pedicle Screw System is intended to provide immobilization and stabilization of spinal segments in skeletally mature patients as an adjunct to fusion for degenerative disc disease, spondylolisthesis, trauma, spinal stenosis, deformities, tumor, and/or ...
Manufactured by:Conformis based inBillerica, MASSACHUSETTS (USA)
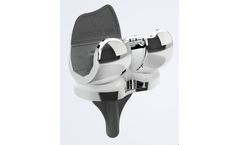
iTotal PS is the only truly custom-made posterior stabilized total knee replacement, designed ...
Manufactured by:Xtant Medical based inBelgrade, MONTANA (USA)
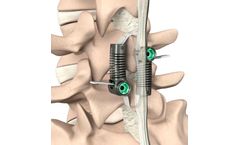
The Axle Interspinous Fusion System is an internal fixation device for posterior spinal surgery in the non-cervical spine (T1-S1 inclusive). It is a minimally invasive, modular Interspinous fusion system with straightforward instrumentation. The Axle Interspinous Fusion System is designed to provide spinal stability for lumbar fusion procedures, including the ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The PerQdisc is designed for use using a lateral traspsoas or retroperitoneal (i.e., anterolateral) surgical approach to access disc space. Access through the annulus can be achieved using the PerQdisc™ Disc Access System (Figure 3). Once annular access is achieved, a nuclectomy (removal of nucleus material) is performed through the disc access using various tools to remove most of the ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The PerQdisc Implant Delivery Device (Figure 4) is then inserted through the Access Cannula into the enucleated disc space. Contrast is injected into the inner chamber of the implant. This aids in visualization as well as provides real-time monitoring of fill pressure. The outer chamber of the implant is then filled under pressure control with a curable, polymer containing barium sulfate. The ...
Manufactured by:ATEC Spine, Inc based inCarlsbad, CALIFORNIA (USA)
Invictus is ATEC’s comprehensive spinal fixation platform, designed to treat a range of pathologies with intraoperative adaptability and surgical predictability through an open, MIS, or hybrid ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The SST PerQdisc Nucleus Replacement System is intended to replace the nucleus pulposus of the intervertebral disc in the L1-S1 spinal region in patients with single-level discogenic pain. The patient may have single or multi-level degenerative disc changes but the discogenic pain must be limited to a single level. The patient must be skeletally mature, at least 21 years of age, presenting with ...
by:Empirical Spine, Inc. based inSan Carlos, CALIFORNIA (USA)
LimiFlex is intended to provide segmental stabilization following decompression for grade 1 lumbar degenerative spondylolisthesis with spinal stenosis. LimiFlex is implanted through a dorsal approach, typically through the same incision used for the surgical ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
The Centurion Posterior Occipital Cervico-Thoracic (POCT) System is a temporary, multiple component system comprised of a variety of non-sterile, single use components made of titanium alloy or cobalt chrome alloy that allow the surgeon to build a spinal implant construct. The Centurion POCT System consists of an assortment of rods, set screws, axial connectors, lateral offset adapters, ...