

- Home
- Companies
- Femasys Inc.
- Products
- FemVue - Saline-Air Device
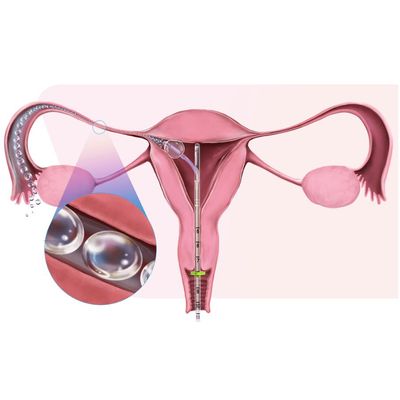
FemVue - Saline-Air Device
FemVue is the first FDA-cleared product that creates natural saline and air contrast and enables safe, reliable, and real time evaluation of the fallopian tubes with ultrasound. When performed with a uterine cavity assessment, a more comprehensive exam can be achieved from the comfort of the GYN’s office.
FemVue is the first FDA-cleared product that delivers natural saline and air as contrast for diagnosis of the fallopian tubes with ultrasound. When paired with FemCath, an FDA-cleared intrauterine catheter, each tube can be selected for individual evaluation. This safe and reliable procedure provides real-time results and can be done along with a uterine cavity assessment for a comprehensive infertility diagnosis.
FemCath, an FDA-cleared intrauterine catheter, is the first to allow for selective evaluation of a fallopian tube with contrast. FemCath features our proprietary delivery platform, which places balloon technology close to the opening of a selected fallopian tube for directed delivery.
FemCerv, an FDA-cleared endocervical sampler, is the first to be designed for capture of a complete and uncontaminated sample in a relatively pain-free procedure. FemCerv features an expandable collection chamber that is exposed during sampling and closed during removal for containment of cervical cells and tissue.
Blocked fallopian tubes account for a large portion of infertility cases.
FemVue creates natural bubbles so your gynecologist can check for tubal infertility (blocked tubes).
Why FemVue
FemVue is a revolutionary device that allows your doctor to easily check your tubes as part of your initial infertility work-up.
- Safe
- Time-Saving
- Affordable
- Convenient
The FemVue Saline-Air Device instills a consistent alternating pattern of saline and air as a continuous stream of contrast medium into the uterus and fallopian tubes to be used in conjunction with an intrauterine catheter for performance of sono-hysterosalpingogram (Sono HSG).
- Any condition that is a contraindication to hysterosalpingography.
- Current or recent pregnancy (previous 6 weeks) including miscarriage which may increase risk of air embolism.
