- Home
- Companies
- Universal Cells Inc.
- Products
- Universal Donor Cells
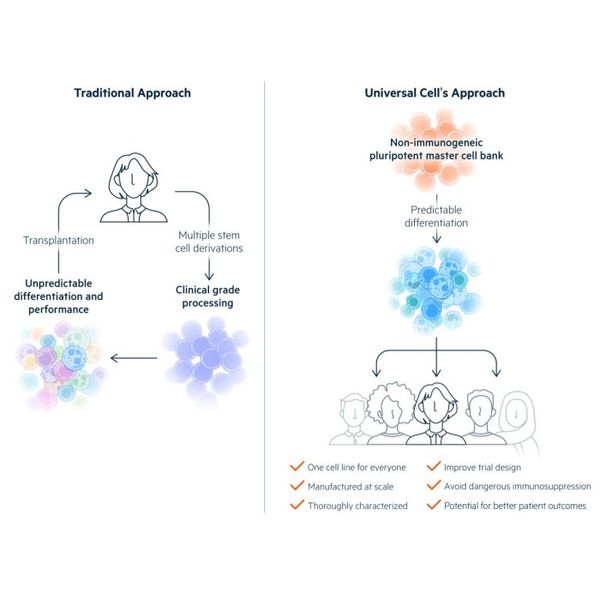
Universal Donor Cells
Realizing the potential of pluripotent stem cells requires that the therapeutic cells derived from PSCs be accepted by patients. PSC-derived cells that have not been engineered to avoid allogeneic rejection will be eliminated soon after transplantation unless they are HLA-matched to their recipients, delivered to immune privileged sites, or administered along with potentially dangerous immunosuppression.
Universal Cells addresses the allogeneic rejection problem by manipulating human leukocyte antigen (HLA) expression in stem cells. In conventional transplantation, multiple HLA class I and class II proteins must be matched for histocompatibility in allogeneic recipients. We eliminate the expression of these polymorphic HLA proteins by gene editing, express specific non-polymorphic HLA molecules to provide essential class I signals that block lysis by Natural Killer cells, and introduce suicide genes for enhanced safety.
A major advantage of Universal Donor Cells (UDCs) is that a single cell line can be differentiated into multiple therapeutic cell products. These Off-the-Shelf products can be tested in clinical trials with the goal of eventual regulatory approval. The aim is for them to be used in any recipient without allogeneic rejection, reducing costs and leading to better patient outcomes.