- Home
- Software
- north america
- clinical metadata governance and trial
Refine by
Applications
- Software Solutions for Patient Groups
- Software Solutions for Researchers
- Software Solutions for Sponsors
- Software Solution for Pharma
- NantHealth healthcare solutions for hospitals and health systems sector
- NantHealth healthcare solutions for practices and medical groups sector
- BrightInsight Solutions for Biopharma
- Software Solution for Patient or Caregiver
- Software Solution for Healthcare Provider
- Software Solution for Payer
- Personal ECG device solutions for biopharma sector
- Supply Solutions for Early-Phase Planning
- RTSM Solutions for Phase 1 Trials
- RTSM Solutions for Phase 2 Trials
- RTSM Solutions for Phase 3 and 4 Trials
- RTSM Solutions for Site satisfaction
Clinical Metadata Governance And Trial Software In North America
104 software items found
by:PointCross Life Sciences Inc. based inFoster City, CALIFORNIA (USA)
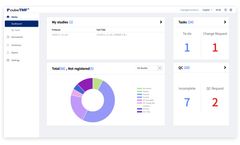
Digital Metadata Governance & Clinical Regulatory Compliance: Management and governance of CDISC and corporate standards driven metadata, data models and study level models from protocol to submission with workflows and controls. Solution for clinical trial data submissions ...
by:Statsol based inBoston, MASSACHUSETTS (USA)
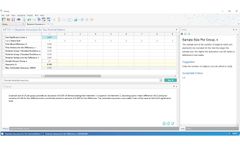
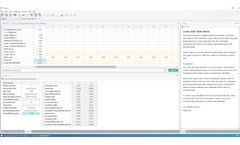
Sample size for Bayesian trial design. Use Bayesian Assurance for better decisions at key clinical trial ...
by:Deep Lens Inc. based inColumbus, OHIO (USA)
VIPER searches the customized matching engine to find the best available clinical trials for a patients’ specific diagnosis—at the time of diagnosis. Through workflow integration, VIPER sends real-time notifications of a patient's eligibility for available clinical trials to the entire care team in the narrow enrollment ...
by:CRScube Inc. based inSeoul, SOUTH KOREA
cubeBUILDER® provides an intuitive interface for sponsors, CROs, and academic institutions to access and rapidly build cubeCDMS®, cubeIWRS®, and cubePRO® system environments to conduct clinical trials electronically and securely on the cloud. cubeBUILDER® is free, easy-to-learn, and ...
Manufactured by:Quantificare Inc. based inSuwanee, GEORGIA (US) (USA)
Forget time-intensive imaging software. Keep your investigators happy and enhance their imaging workflow with a comprehensive web solution. DermaPix® Web Portal is the end-to-end solution for the upload, management, monitoring and real-time review of digital images in multicenter clinical ...
by:Cytel based inWaltham, MASSACHUSETTS (USA)
East Bayes provides easy access to complex Bayesian designs for all users. Cytel is bringing you East Bayes as a web-based extension of East for clinical trial design that blends the pace of SaaS delivery, the ease of use and robustness of Cytel software, and the velocity of cloud-based ...
by:Clinion based inHyderabad, INDIA
Clinion's Electronic Trial Master File (eTMF) software optimizes clinical trial management with secure, efficient, and compliant document ...
by:Statsol based inBoston, MASSACHUSETTS (USA)
Adaptive trials can reduce the length of your trial by up to ...
by:Statsol based inBoston, MASSACHUSETTS (USA)
Accurately predict your key trial milestones. Identify roadblocks and take action to keep your trial on ...
by:Cytel based inWaltham, MASSACHUSETTS (USA)
The use of independent Data Monitoring Committees (DMCs) for clinical trials has grown dramatically across all therapeutic areas. As such practice continues to expand, sponsors face increased pressure to form committees that ensure adherence to charters, study protocols and regulatory requirements. ACES provides support to DMCs by offering a web-based trial workflow and document management ...
by:CRScube Inc. based inSeoul, SOUTH KOREA
In the paperless era, the clinical trial industry is changing. Until now, the Trial Master File (TMF) has consisted of traditional paper documents, images, or media organized in a binder stored in file cabinets. In accordance with FDA's CFR 21 Part 11, we have developed cubeTMF to provide a validated system for a cohesive workflow for a truly paperless clinical trial. cubeTMF® streamlines the ...
by:Medidata based inNew York, NEW YORK (USA)
Medidata eCOA (Clinical Outcome Assessment) is revolutionizing the way sponsors, CROs, and sites collect electronic data from patients, physicians, and caregivers. Available as an iOS or Android app or web-based solution, Medidata eCOA provides a flexible, intuitive model for capturing patient data that is designed to make it easier for patients to engage in clinical trials. Built as part of the ...
by:Simulations Plus based inLancaster, CALIFORNIA (USA)
MonolixSuite is a comprehensive software suite designed for model-informed drug development, particularly focused on pharmacometrics. It provides a fast and user-friendly environment for analysis, modeling, and simulation, enabling full integration of applications for a seamless workflow. The suite encompasses capabilities ranging from data visualization to non-compartmental and population ...
by:BrightInsight, Inc based inSan Jose, CALIFORNIA (USA)
Accelerate your time to market and reduce development costs with our pre-built Patient App and Clinician ...
by:Statsol based inBoston, MASSACHUSETTS (USA)
nQuery is the world’s most trusted clinical trial design platform. In 2021, 88% of organizations with FDA approved clinical trials used ...
by:Endpoint Clinical based inWakefield, MASSACHUSETTS (USA)
Simplify your data life cycle to enable alignment and unlock efficiency across your clinical ...
by:intelligencia.ai based inNew York, NEW YORK (USA)
Assess the probability of technical and regulatory success (PTRS) and phase transition probability of your pipeline programs in an objective and efficient way. Understand the supporting rationale, track the evolution of PTRS based on key events, and make portfolio and program-level decisions. ...
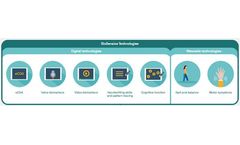
Manufactured by:BioSensics LLC based inNewton, MASSACHUSETTS (USA)
An integrated solution for centralized and objective assessment of gait, balance, motor symptoms, speech and disease rating scales during site ...
by:Clinion based inHyderabad, INDIA
Our Electronic Data Capture Software Making Clinical Trials Faster and Better. Embrace Integration: Integrate Clinion Electronic Data Capture Software with Clinion CTMS, IWRS/RTSM, and ePRO/eCOA for true end-to-end support of your Study, obviating the hassles of data reconciliation while dealing with multiple eClinical ...
Manufactured by:AliveCor, Inc based inMountain View, CALIFORNIA (USA)
KardiaRx is an iOS/Android app which enables remote ECG capture and monitoring in decentralized clinical trials. When the app pairs with KardiaMobile 6L, patient ECGs are automatically uploaded to KardiaPro, our web-based portal, or another preferred EDC platform - for 24/7 access to critical cardiac rhythm ...