Refine by
Cell And Genetic Therapies Equipment & Supplies
306 equipment items found
Premium
Manufactured by:AB Sciex LLC based inFramingham, MASSACHUSETTS (USA)
Perform consistent and compliant biotherapeutics characterization faster than ever. With the PA 800 Plus system, you can confidently safeguard the success of your biologics. Run multiple characterizations from a single system that biopharma labs depend on, and produce qualitative and quantitative analyses, with speed and ...
Manufactured by:Xcell Biosciences Inc. based inSan Francisco, CALIFORNIA (USA)
Cell therapy manufacturing re-imagined. An automated, closed system for the production of autologous cell therapies. The KALI Cell Foundry is currently available for licensing ...
Manufactured by:AD.TECH – Albian Equipment based inBilbao, SPAIN
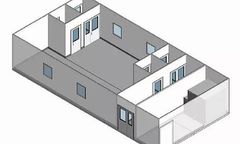
A physical barrier between a process that may require sterility and pressurisation, either positive or negative, from the outside environment, ensuring the necessary protection, reducing potential contamination and risks of cross-contamination and achieving the required air quality in the respective areas. ...
Manufactured by:G-CON Manufacturing, Inc. based inCollege Station, TEXAS (USA)
G-CON has developed a portfolio of six standardPOD Cleanrooms. These PODs are fully designed, allowing them to be mass-produced. Delivery time is as little as 3 months with the future goal of being able to maintain an inventory of ready to deliver PODs. Mass production of standardPODs will also lower costs. We believe cleanroom infrastructures have to become an off-the-shelf piece of ...
Manufactured by:Ardigen based inKraków, POLAND
Accelerate discovery and improve safety of TCR therapies using Artificial Intelligence. Following the unique opportunity for curing patients provided by the development of cell therapies (e.g. TCR discovery), Ardigen has set on the path to advance the field with its Artificial Intelligence platform. Many challenges stand in the way of successful therapy discovery and development. Let us know how ...
Manufactured by:Obsidian Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
Engineered with membrane-bound IL15, Eliminates toxic IL2 regimen, increasing patient accessibility to TIL therapy, Drives improved persistence, IL15 expression controlled by acetazolamide (ACZ), via cytoDRiVE® ...
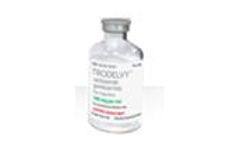
Manufactured by:Gilead based inFoster City, CALIFORNIA (USA)
Sacituzumab govitecan-hziy 180 mg for injection. ...
by:Chimeric Therapeutics based inCarlton South, AUSTRALIA
CHM 3301 (Undisclosed CAR NK) will be developed on the next generation CORE-NK platform leveraging a currently undisclosed Chimeric Antigen ...
by:Chimeric Therapeutics based inCarlton South, AUSTRALIA
CHM 2301 (CDH17 CAR NK) will be developed on the next generation CORE-NK platform leveraging our CDH17 Chimeric Antigen ...
Manufactured by:Orgenesis Inc. based inGermantown, MARYLAND (USA)
We leverage closed, automated processes that are validated for reliability and ...
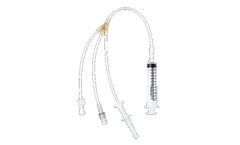
Manufactured by:Charter Medical, LLC based inWinston-Salem, NORTH CAROLINA (USA)
Charter Medical’s Advect® Fluid Transfer Sets are designed to support the quality and manufacturing requirements of personalized care. The transfer sets are intended for sterile transfer of bone marrow mononuclear cells from one container to another. ...
Manufactured by:GeminiBio based inWest Sacramento, CALIFORNIA (USA)
GemCell Plus Xeno-Free Human Serum AB enables advanced therapy researchers and developers to streamline their workflow, allowing them to bring their end products to market quickly by providing human serum that is scalable, free of bovine components, uses therapeutic-grade human thrombin, and is rigorously ...
Manufactured by:Polysciences, Inc. based inWarrington, PENNSYLVANIA (USA)
MAXgene GMP Transfection Reagent is a cGMP transfection reagent for the development and manufacturing of viral vectors for cell-and gene-based therapies. It is an ideal reagent in HEK293 and CHO systems for the manufacture of AAVs, LVs and recombinant proteins. MAXgene GMP capitalizes on the efficiency and scalability of Polysciences’ PEI MAX while adding the validation process and ...
by:Caribou Biosciences, Inc. based inBerkeley, CALIFORNIA (USA)
Our next-generation chRDNA genome-editing platform is the foundation for the development of differentiated allogeneic cell therapies. Our chRDNA technology enables multiple genome edits, including multiplex gene insertions, with a high degree of specificity and lower levels of off-target editing than first generation CRISPR-Cas9. ...
by:Chimeric Therapeutics based inCarlton South, AUSTRALIA
CHM 1301 (CLTX CAR NK) will be developed on the next generation CORE-NK platform leveraging our CLTX Chimeric Antigen ...
by:Chimeric Therapeutics based inCarlton South, AUSTRALIA
The next generation CORE-NK platform will integrate additional activation and expansion features to further optimize the cancer-fighting power of the CORE-NK platform cells. Development of the next generation CORE-NK platform will be as a combination therapy for blood ...
by:bit.bio based inCambridge, UNITED KINGDOM
opti-ox™ is capable of producing batches of every cell in the human body at scale and gives us the power to precision engineer human cells. This is because it reliably activates specific transcription factors within the cells. Transcription factors tell a cell what to do because they control the programs (gene regulatory networks) of the cell. Together, these form the operating system of ...
by:Cytori Therapeutics Inc. based inSan Diego, CALIFORNIA (USA)
The Celution System is a medical technology developed by Cytori to automate and standardize the extraction and concentration of adult Adipose-Derived Regenerative Cells (ADRCs) in a clinical setting. The Celution System enables real-time access to autologous, clinical-grade ADRCs at the point-of-care facilitating cell therapy through the reimplantation or reinfusion of a patient’s own ...
Manufactured by:Altucell, Inc. based inNew York, NEW YORK (USA)
Encapsulated cell therapy is an emerging area of biopharmaceutical research that aims to unleash the therapeutic potential of cells to treat serious diseases without the need for immunosuppression. Altucell has developed patented, proprietary engineered cells that are combined with a patented encapsulation technology to create effective ...
by:Genmab A/S based inCopenhagen V, DENMARK
Ofatumumab is a human monoclonal antibody which targets an epitope in the CD20 molecule encompassing parts of the small and large extracellular ...