Show results for
Refine by
Icellator Clinically Studied Equipment Supplied In Usa
2 equipment items found
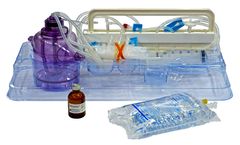
Manufactured by:Tissue Genesis based inNew Albany, INDIANA (USA)
The Icellator2 system set the standard, providing SVF cell yields in 65 minutes. Currently approved for use in The Bahamas and available in Japan. In the USA, clinical FDA-approved IDE studies using the Icellator® are underway for a variety of ...
Manufactured by:Tissue Genesis based inNew Albany, INDIANA (USA)
Class III approval for commercial use by the South Korean Ministry of Food and Drug Safety (MFDS). Approved for clinical use in South Korea & The Bahamas. In the USA, FDA-approved clinical IDE studies using the Icellator and Cell Icellation Kit are underway for multiple indications. Produced under ISO 13485 according to 21 CFR 820 ...