- Home
- Equipment
- north america
- igg igm test kit
Refine by
Igg Igm Test Kit Equipment Supplied In North America
10 equipment items found
Distributed by:Labex Canada Inc. based in, ONTARIO (CANADA)
Coronaviruses belong to a large family of potentially harmful RNA viruses. In humans, they are known to be involved mainly in respiratory infections ranging from the common colds to more severe diseases such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). The recently discovered coronavirus, SARS-CoV-2, causes coronavirus disease COVID-19, a significant ...
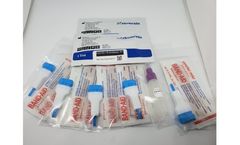
Manufactured by:Spark Mobile Diagnostics based inJamnagar, INDIA
Spark Diagnostics is pleased to introduce a Rapid Whole Blood Test for COVID-19IgG / IgM: Cov-CHECK. The test is designed for the rapid qualitative detection of IgG and IgM antibodies to the 2019 novel Coronavirus (2019-nCoV,SARS-CoV-2) in human whole blood, serum or plasma. Cov-CHECK™ – COVID-19 IgG / IgM Test Device is a supplemental rapid screening tool for symptomatic or ...
Manufactured by:ACON Laboratories, Inc. based inSan Diego, CALIFORNIA (USA)
Qualitative detection of Hepatitis B Core Antibody (HBcAb) in serum or ...
Manufactured by:ACON Laboratories, Inc. based inSan Diego, CALIFORNIA (USA)
Qualitative and quantitative detection of Helicobacter pylori (H. pylori) Antigen in ...
Manufactured by:ACON Laboratories, Inc. based inSan Diego, CALIFORNIA (USA)
Qualitative and quantitative detection of IgG antibodies to Helicobacter pylori (H. pylori) in serum or ...
Manufactured by:TBG Diagnostics Limited (TDL) based inMelbourne, AUSTRALIA
The SARS-CoV-2 IgG/IgM Rapid Test Kit is used to qualitatively detect IgG and IgM antibodies of SARS-CoV-2 in human serum, plasma or whole blood. This device is intended to be used by professionals as a screening test and for SARS-CoV-2 research. Any reactive specimen with the ...
Manufactured by:America Diagnostics, Inc. based inSan Diego, CA 92126 USA, CALIFORNIA (USA)
This product is for in vitro diagnostic use, following guidance from the FDA for Emergency Use Authorizations of tests submitted for approval on March 16, 2020. This test has not been reviewed by the FDA and results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection ...
Manufactured by:America Diagnostics, Inc. based inSan Diego, CA 92126 USA, CALIFORNIA (USA)
This product is for in vitro diagnostic use, following guidance from the FDA for Emergency Use Authorizations of tests submitted for approval on March 16, 2020. This test has not been reviewed by the FDA and results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection ...
Manufactured by:ACON Laboratories, Inc. based inSan Diego, CALIFORNIA (USA)
The SARS-CoV-2 IgG and IgM EIA Test Kits are qualitative enzyme immunoassays for the detection of IgG or IgM antibodies to SARS-CoV-2 in human serum or ...
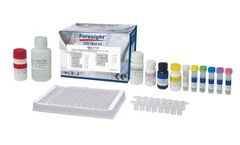
Manufactured by:Nirmidas Biotech, Inc. based inPalo Alto, CALIFORNIA (USA)
The test can be used for a variety of samples including serum, plasma, whole blood, dry blood spot, and saliva in a lab facility. It can be used to assess IgG and IgM levels in a sample with 100% sensitivity and 99.87% specificity without interferences from other diseases. Avidity pGOLD™ assay can tell if a COVID-19 infection is recent or remote greater than 6 months ...