- Home
- Equipment
- north america
- synthetic bone
Show results for
Refine by
Synthetic Bone Equipment Supplied In North America
18 equipment items found
Manufactured by:Inion Oy based inTampere, FINLAND
Inion BioRestore™ is a synthetic bone graft substitute, which remodels into bone and is easy to use. Inion BioRestore™ is made of degradable bioactive glass, which once in contact with the natural body fluids forms a silica gel and calcium phosphate layer, providing scaffolding for the new bone tissue to be ...
Manufactured by:Parametrics Medical based inLeander, TEXAS (USA)
Synthetic bone void fillers are safe, biocompatible, and easy to use. These resorbable biomaterials are based on nano-crystalline hydroxyapatite (HA) and purified collagen technologies. These products are engineered to optimize the resorption rate. Our granules are composed of 60% HA and 40% β-Tricalcium Phosphate (TCP). The formulation provides optimal ...
Manufactured by:Surgentec LLC based inBoca Raton, FLORIDA (USA)
OsteoFlo NanoPutty is the world’s first, and only quadphasic synthetic bone graft putty with nano-surface technology that allows for advanced bone healing. OsteoFlo® NanoPutty® has been designed to provide true nanobiology with an optimal resorption profile and superior handling characteristics. This novel synthetic ...
Manufactured by:Life Spine, Inc. based inHuntley, ILLINOIS (USA)
Life Spine’s Graft Delivery Device is engineered to deliver allograft, autograft or synthetic bone graft materials directly to an orthopedic surgical site ...
Manufactured by:Xtant Medical based inBelgrade, MONTANA (USA)
The SimpliMix™ Graft Delivery Device is designed for targeted delivery of hydrated allograft, autograft, or synthetic bone graft materials to an orthopedic surgical site, while maximizing material ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
Bonus Synthetic Bone Graft Substitute granules are resorbable, osteoconductive matrices consisting of a thin, 2–10 micron layer of hydroxyapatite over a calcium carbonate core. Bonus Synthetic Bone Graft Substitute has been indicated as a bone graft substitute that resorbs and is replaced with ...
Manufactured by:OrthoPediatrics Corp. based inWarsaw, INDIANA (USA)
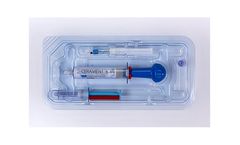
CERAMENT BONE VOID FILLER is an injectable, moldable and drillable synthetic bone void filler consisting of 40% hydroxyapatite, 60% calcium sulfate and the radio-contrast agent iohexol. The unique ratio of hydroxyapatite and calcium sulfate is designed to enable CERAMENT to resorb at the same rate that bone forms. Calcium sulfate ...
by:Molecular Matrix, Inc. based inWest Sacramento, CALIFORNIA (USA)
Osteo-P™ Bone Graft Substitute (BGS) is a non-mineralized, synthetic bone void filler consisting of a hyper-crosslinked carbohydrate polymer. It is highly porous, biocompatible, biodegradable, and osteoconductive. Osteo-P™ BGS is intended for the filling of bone defects created surgically or as a result of traumatic ...
by:In2Bones Global based inMemphis, TENNESSEE (USA)
The i2b Resorbable Bead Kit is designed to create easy-to-use bio-resorbable beads for filling osseous defects. i2b® Resorbable Bead Kits allow surgeons to cast 100% pure synthetic resorbable bone void filler Beads in two sizes: 3.5 and 4.5mm. The beads provide a safe alternative to human or animal cadaver bone that completely eliminates the ...
Manufactured by:True Phantom Solutions Inc. (TPS) based inWindsor, ONTARIO (CANADA)
Adult Ear Phantom for R&D is a product with precise anatomy of a human ear and it can be used for research and development of noise reduction equipment. It can be fabricated as a stand-alone product or implemented to the full skull and/or ...
Manufactured by:True Phantom Solutions Inc. (TPS) based inWindsor, ONTARIO (CANADA)
Adult Human Torso for R&D is a realistic medical imaging phantom that can be customized both internally & externally to suit any research application. It can be designed and produced to be compatible with Ultrasound, X-RAY, CT, and ...
Manufactured by:Isto Biologics based inHopkinton, MASSACHUSETTS (USA)
InQu® is the cell-friendly biosynthetic™ bone graft with proven clinical efficacy leading to faster bone ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
BioSphere Flex is a bone graft that was specifically developed to maximize the bone healing potential of bioactive glass. Using Synergy’s proprietary BioSphere Technology, BioSphere Flex is composed of innovative bioactive glass granules combined with a porous collagen/sodium hyaluronate ...
Manufactured by:MDBiologix based inUnionville, ONTARIO (CANADA)
Marrow Cellution™ bone graft kits provide high quality bone marrow aspirate and cancellous bone autograft, collected from numerous sites within the marrow space – achieving the gold standard of autograft in a minimally invasive ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
βBeta-bsm is injectable osteoconductive material for use in minimally invasive procedures. This synthetic, biocompatible bone graft substitute material sets hard and maintains shape at body temperature. The material is mixed in a closed syringe-to-syringe mixing system to minimize the potential for spills, and can be delivered through a needle as small as ...
by:RevBio Inc. based inLowell, MASSACHUSETTS (USA)
Tetranite is a gamechanger for clinicians striving to improve outcomes across the range of human and animal bone repair applications. Currently there is no FDA approved biomaterial with the adhesive, osteo-conductive, and bioresorbable qualities that Tetranite possesses—all qualities which have been proven in existing pre-clinical animal studies. Tetranite is poised to ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
Designed to process a mixture of autologous whole blood and bone marrow aspirate, the BioCUE BBMA Concentration System represents an evolution in this technique. The system includes all the components to DRAW blood, ASPIRATE bone marrow, easily PROCESS the disposable system, and produce an autologous PRP output to HYDRATE the surgeon’s choice of autograft and/or allograft ...
by:NuShores Biosciences LLC based inLittle Rock, ARKANSAS (USA)
Today there is no satisfactory solution for major bone injuries caused by accidents, warfare and cancer, especially complex and large segmental bone loss. Our NuCress™ bone scaffold technology has been shown to promote healing of large segmental bone breaks (>2.5 cm) tested in large and small animals (goats, mice) – with no infections, inflammation, rejection or adverse bone ...