- Home
- Equipment
- usa california
- therapy device
Refine by
Applications
- Acute Kidney Injury (AKI) Device for Reduction of Galectin-3 Levels
- Acute Kidney Injury (AKI) Device for Plasmapheresis Therapy
- Acute Kidney Injury (AKI) Device for Patient Selective Apheresis
- Intraoperative Radiation Therapy (IORT) System - Electron Therapy for Skin Cancer
- Intraoperative Radiation Therapy (IORT) System for Flash Radiotherapy
- Intraoperative Radiation Therapy (IORT) System for Pancreatic Cancer Treatment
- Intraoperative Radiation Therapy (IORT) System for Breast Cancer Treatment
- Intraoperative Radiation Therapy (IORT) System for Single-Fraction APBI
- Intraoperative Radiation Therapy (IORT) System for Surgical Oncologists
- Intraoperative Radiation Therapy (IORT) System for Radiation Oncologists
- Intraoperative Radiation Therapy (IORT) System for Hospital Administrators
Therapy Device Equipment Supplied In Usa California
38 equipment items found
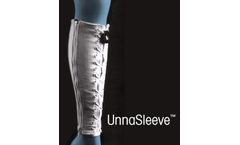
Manufactured by:ACI Medical, LLC based inSan Marcos, CALIFORNIA (USA)
True Non-Stretch Compression Therapy: Adjustable, reusable and washable; Easy to apply & remove; In-office fitting in seconds; Time & cost savings over conventional Unna’s ...
Manufactured by:Cala Health, Inc. based inBurlingame, CALIFORNIA (USA)
Cala Trio therapy is a groundbreaking treatment designed to temporarily and effectively reduce your hand tremor. It is the first and only non-invasive therapy that directly targets the source of the tremor by gently stimulating the wrist nerves with electricity (neuromodulation), and targeting the root cause of essential tremor. The Cala Trio ...
Manufactured by:AMREX, Trademark of Amrex-Zetron Inc. based inParamount, CALIFORNIA (USA)
The MS324C is designed for the application of low volt ac muscle stimulation. The MS324C is dual channel, four pad, low voltage electrical muscle stimulator that produce pulsation, tetanize, surge, and reciprocal output. The MS324C features the patient treatment stop switch. Designed to promote patient assurance, the treatment stop switch allows the patient to discontinue stimulator output at the ...
Manufactured by:Novartis International AG based inBasel, SWITZERLAND
Our investigational gene therapy, GT005, is designed to restore balance to a part of our immune system called the complement ...
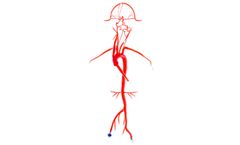
Manufactured by:United Biologics, Inc. based inIrvine, CALIFORNIA (USA)
Our Neuro System Trainer extends from the femoral arteries to the pericallosals and A2, M2 segments, and features six aneurysms of varying sizes. The left and right vertebrals are customized to simulate both a young and mature adult. To secure your vessel during testing or demonstrations, we offer 3D/2D Stabilizing Platforms. Benefits include a non-slip surface, easy access, and clear, adjustable ...
Manufactured by:Advanced Oxygen Therapy Inc. based inOceanside, CALIFORNIA (USA)
The TWO2 therapy Multi-Patch system is comprised of two major components: A single use Multi-Patch chamber and an oxygen source. An oxygen flow rate of ≈ 4 lpm applied into the chamber delivers 30 mb of non-contact pressure directly to the wound site, within a sealed and humidified environment. The resultant oxygen partial pressure diffusion gradient of close to 800 mmHg provides for ...
Manufactured by:EBR Systems, Inc. based inSunnyvale, CALIFORNIA (USA)
The WiSE CRT System was developed to address the persistent limitations of current CRT systems and to provide a more customized, patient-specific ...
Manufactured by:MedSpark, LLC based inSan Luis Obispo, CALIFORNIA (USA)
However, several developmental disabilities can inhibit this process, including Cerebral Palsy, Spina Bifida, Downs Syndrome, Traumatic Brain Injury, and various physical abnormalities. Many therapeutic crawling devices exist to aid physical therapists and caregivers in assisting these children during therapy. However, the current devices are ...
Manufactured by:Hitachi America, Ltd. based inSanta Clara, CALIFORNIA (USA)
Hitachi provides a Hybrid particle therapy system which can irradiate both proton and carbon-ion from one ...
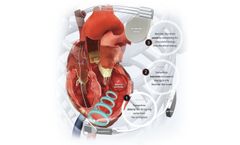
Manufactured by:Ancora Heart, Inc based inSanta Clara, CALIFORNIA (USA)
As the only completely transcatheter therapy to treat the enlarged left ventricle, the AccuCinch Ventricular Restoration System is a fundamentally different and innovative device-based therapy designed to improve the structure and function of the heart. For heart failure patients with reduced ejection fraction (HFrEF) who remain symptomatic ...
Manufactured by:Eliaz Therapeutics based inSanta Rosa, CALIFORNIA (USA)
Despite efforts to develop new interventions, current therapeutic options are limited in scope and effectiveness. At Eliaz Therapeutics, we have developed a patented, unique medical device that will remove Galectin-3 (Gal-3) from human blood. Our device removes both bound and free Gal-3 from the circulation, offering a new treatment option that is less invasive ...
Manufactured by:Jant Pharmacal Corporation based inEncino, CALIFORNIA (USA)
The Coag-Sense Prothrombin Time (PT)/INR Monitoring System is an in vitro diagnostic device that provides quantitative prothrombin time (PT) results, expressed in seconds and INR units. It uses fresh capillary whole blood. It is intended for use by health care professionals at the point of care to monitor patients who are on warfarin-type (coumarin) anticoagulation ...
Manufactured by:Biocardia, Inc. based inSunnyvale, CALIFORNIA (USA)
Where the CardiAMP Cell Therapy System uses a patient’s own cells, the CardiALLO system uses culture-expanded, bone marrow-derived mesenchymal stem cells from a universal donor. This allows the cells to be obtained "off the shelf" from a pharmacy, negating the need for a bone marrow aspirate for the patient and providing an option for patients identified as sub-optimal for the CardiAMP ...
Manufactured by:LinaTech based inShanghai, CHINA
TiGRT DICOM RTLink system (RTLink) is mainly for users with multiple sets of TPS’s or linacs. RTLink provides data connection for radiotherapy, and also solutions for data transfer and interaction management between different devices. TiGRT RTLink can be used to connect most radiotherapy devices, like medical imaging equipments (CT, MRI, PET), treatment planning system (TPS), doctor ...
Manufactured by:Advanced Sterilization Products (ASP) based inIrvine, CALIFORNIA (USA)
CIDEX® OPA Solution is a high-level disinfectant for reprocessing reusable heat-sensitive semi-critical medical devices such as endoscopic, respiratory therapy, and anesthesia equipment. CIDEX® OPA Solution is a high-level disinfectant for reprocessing reusable heat-sensitive semi-critical medical devices such as endoscopic, respiratory ...
by:Accuray based inSunnyvale, CALIFORNIA (USA)
The CyberKnife® S7™ stereotactic radiosurgery (SRS) and stereotactic body radiation therapy (SBRT) system empowers clinicians to confidently deliver personalized treatments to more patients. As the only robotic radiosurgery system that can deliver non-surgical stereotactic treatments with sub-millimeter accuracy, anywhere in the body — including the prostate, lung, brain, spine, ...
Manufactured by:RSD Radiology Support Devices based inLong Beach, CALIFORNIA (USA)
The Alderson Radiation Therapy (ART) Phantom is a refined and improved version of the Alderson RANDO Phantom in both design and materials. ART Phantoms are designed within highly sophisticated technological constraints and follow ICRU-44 standards. Additionally, they provide integrated tests of the entire chain of treatment planning and delivery. ART Phantoms are molded of tissue-equivalent ...
Manufactured by:PAVmed Inc. based inNew York, NEW YORK (USA)
The Oncodisc device is an intelligent implantable vascular access device embedded with advanced sensor and communication technology. In addition to delivery of pharmaceutical therapy, the device reliably and accurately monitors patient physiology for early detection of illness or treatment-related complications. ...
Manufactured by:Ivantis, Inc. based inIrvine, CALIFORNIA (USA)
Roughly the size of an eyelash, the highly flexible Hydrus Microstent is placed by a doctor using microscopic incisions and advanced equipment. This less invasive approach allows for fewer complications and faster healing times than traditional glaucoma ...
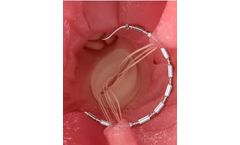
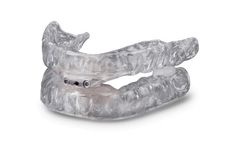
Manufactured by:Glidewell based inNewport Beach, CALIFORNIA (USA)
Helping patients who suffer from sleep-disordered breathing, the TAP 3 TL is part of the TAP family of appliances for the treatment of snoring and obstructive sleep apnea (OSA). Based on the same principle as cardiopulmonary resuscitation (CPR), the TAP (Thornton Adjustable Positioner) was designed to keep the airway open to allow for air to pass. A constricted or collapsed airway causes snoring ...