- Home
- Equipment
- north america
- tissue replacement
Refine by
Tissue Replacement Equipment Supplied In North America
23 equipment items found
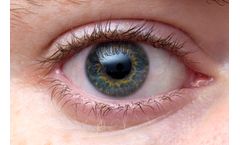
Manufactured by:Bio-Tissue, Inc. based inMiami, FLORIDA (USA)
AmnioGraft is a biologic ocular transplantation graft used by eye surgeons around the world as an adjunct therapy to treat ocular surface indications such as corneal ulcers, pterygium, mechanical dry eye (also known as conjunctivochalasis), excision of tumors, chemical burns, and Stevens-Johnson Syndrome. AmnioGraft serves as a tissue replacement by delivering ...
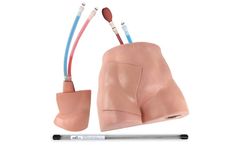
Manufactured by:Simulab Corporation based inSeattle, WASHINGTON (USA)
The Common Bile Duct Exploration Model is a realistic model for the practice of laparoscopic transcystic common bile duct exploration. This model includes a soft tissue base with anatomical landmarks and one replaceable tissue. It is ideal for practicing laparoscopic transcystic common bile duct exploration (LAPTC-CBDE), laparoscopic ...
Manufactured by:Integra LifeSciences based inPrinceton, NEW JERSEY (USA)
AmnioExcel® Amniotic Allograft Membrane is a human placental-based tissue product processed using a proprietary method. The membrane acts as protective covering when placed over the wound, providing the key components found in human amnion including an intact extracellular matrix (ECM), growth factors and cytokines. AmnioExcel easily molds and conforms to the wound, and helps ...
Manufactured by:Simulab Corporation based inSeattle, WASHINGTON (USA)
Learn complete airway management from nasal and oral intubation - to needle and surgical cricothyroidotomy. AirwayMan offers low cost, replaceable soft tissue components for increased realism and extended product life. Every part of AirwayMan is carefully engineered to recreate the familiar movements, textures and delicate nuances of human anatomy. AirwayMan ...
Manufactured by:Simulab Corporation based inSeattle, WASHINGTON (USA)
Simulab 's Ultrasound Arterial Line and ABG Trainer allows educators to train ultrasound-guided radial artery catheterization and perform ABG sampling. This trainer brings medical simulation to new levels with variable pulse strength and rate, palpable landmarks, sharp ultrasound imaging, and arterial pressure that fills an ABG sampling syringe. With this true-to-life hand and arm model, users ...
Manufactured by:Simulab Corporation based inSeattle, WASHINGTON (USA)
Simulab's AirwayChild Pediatric Airway Management Training System is a pediatric airway management trainer simulating a 5-year old child. The soft-tissue trainer has realistic anatomy for an immersive training experience. Train oral and nasal intubation, as well as cricothyroidotomy. The AirwayChild is ultra-realistic, yet affordable and portable. ...
Manufactured by:Bio-Tissue, Inc. based inMiami, FLORIDA (USA)
AmnioGuard is an umbilical cord graft used by Oculoplastic surgeons for several indications. Due to its high tensile strength and thickness, AmnioGuard is easy to handle and suture and can be used for corneal, conjunctival, sclera, fornix, socket, and eyelid ...
Manufactured by:Simulab Corporation based inSeattle, WASHINGTON (USA)
Simulab's AirwayChild® Pediatric Airway Management Training System is a pediatric airway management trainer simulating a 5-year old child. The soft-tissue trainer has realistic anatomy for an immersive training experience. Train oral and nasal intubation, as well as cricothyroidotomy. The AirwayChild is ultra-realistic, yet affordable and portable. Included in this package ...
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
An extracellular matrix derived from porcine small intestine submucosa (SIS), ProxiCor for Cardiac Tissue Repair (CTR) regulates the biologic healing response to decrease inflammation and stimulate formation of healthy tissue.2,3 When sutured next to healthy, viable tissue, ProxiCor is gradually replaced with native ...
Manufactured by:Simulab Corporation based inSeattle, WASHINGTON (USA)
Simulab's AirwayChild Pediatric Airway Management Training System is a pediatric airway management trainer simulating a 5-year old child. The soft-tissue trainer has realistic anatomy for an immersive training experience. Train oral and nasal intubation, as well as cricothyroidotomy. The AirwayChild is ultra-realistic, yet affordable and portable. Included in this package are an ...
Manufactured by:Creative Bioarray based inShirley, NEW YORK (USA)
These properties in combination with their developmental plasticity have generated tremendous interest in the potential use of mesenchymal stem cells to replace damaged tissues. MSC cultured without serum in the presence of transformation growth factor will differentiate into chondrocytes, whereas MSC cultured in serum with ascorbic acid and dexamethasone will ...
Manufactured by:Bio-Tissue, Inc. based inMiami, FLORIDA (USA)
AmnioGraft is a biologic ocular transplantation graft used by eye surgeons around the world as an adjunct therapy to treat ocular surface indications such as corneal ulcers, pterygium, mechanical dry eye (also known as conjunctivochalasis), excision of tumors, chemical burns, and Stevens-Johnson ...
Manufactured by:Cornea Biosciences, Inc. based inRancho Santa Margarita, CALIFORNIA (USA)
The World Health Organization estimates over 10 million people in the world are blind in one or both eyes from corneal injury or disease and up to 45 million people could benefit from corneal transplants. According to data from eye banks and government health agencies, less than 150,000 corneal transplants are done annually worldwide due to a shortage of human cadaver corneas. ...
Manufactured by:Vascudyne, Inc. based inStillwater, MINNESOTA (USA)
Tissue patches are routinely used in surgery for soft tissue repair and reconstruction throughout the body in cardiovascular, orthopedic, abdominal and thoracic wall applications. Current therapies are predominantly xenogeneic, allogeneic or synthetic biomaterials such as bovine pericardium, ePTFE, small intestinal submucosa, and dermal grafts. However, issues persist: incomplete tissue ...
Manufactured by:Simulab Corporation based inSeattle, WASHINGTON (USA)
Simulab's FemoraLineMan System is an ultrasound-compatible femoral line vascular access trainer. The product offers an effective training solution for central venous or arterial access at the femoral site. ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The PerQdisc is designed for use using a lateral traspsoas or retroperitoneal (i.e., anterolateral) surgical approach to access disc space. Access through the annulus can be achieved using the PerQdisc™ Disc Access System (Figure 3). Once annular access is achieved, a nuclectomy (removal of nucleus material) is performed through the disc access using various tools to remove most of the ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The PerQdisc Implant Delivery Device (Figure 4) is then inserted through the Access Cannula into the enucleated disc space. Contrast is injected into the inner chamber of the implant. This aids in visualization as well as provides real-time monitoring of fill pressure. The outer chamber of the implant is then filled under pressure control with a curable, polymer containing barium sulfate. The ...
Manufactured by:Simulab Corporation based inSeattle, WASHINGTON (USA)
Simulab's FemoraLineMan Training Package is an ultrasound-compatible task trainer that offers an effective training solution for central venous or arterial access using the femoral site. This femoral line trainer uses the same patented technology as the highly acclaimed TraumaMan System and allows medical professionals to train using real-time ultrasound guidance during venous or arterial ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The SST PerQdisc Nucleus Replacement System is intended to replace the nucleus pulposus of the intervertebral disc in the L1-S1 spinal region in patients with single-level discogenic pain. The patient may have single or multi-level degenerative disc changes but the discogenic pain must be limited to a single level. The patient must be skeletally mature, at least 21 years of age, presenting with ...
Manufactured by:Allergan Aesthetics based inIrvine, CALIFORNIA (USA)
Breast implants are not considered lifetime devices. The longer people have them, the greater the chances are that they will develop complications, some of which will require more surgery. Breast implants have been associated with the development of a cancer of the immune system called breast implant–associated anaplastic large cell lymphoma (BIA-ALCL). This cancer occurs more commonly in ...