Show results for
Refine by
Applications
- Technology for Surface Modification and Drug Delivery
- Ultrasound for Anesthesiology
- Ultrasound for Cardiology
- Ultrasound for Critical Care
- Ultrasound for Emergency Medicine
- Ultrasound for EMS
- Ultrasound System for Breast Cancer Patients
- First Rapid Infuser for Sepsis
- First Rapid Infuser for Hypotension / Shock
- First Rapid Infuser for EMS/Military
- First Rapid Infuser for Pediatric
- First Rapid Infuser for Research Grants
Vascular Access Equipment & Supplies In In Hungary
90 equipment items found
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
The Ellipsys® Vascular Access System provides a groundbreaking, minimally invasive and cost effective method to create a percutaneous arteriovenous (AV) fistula for hemodialysis access in patients with End-Stage Renal ...
Manufactured by:Hemodia SAS based inLabège, FRANCE
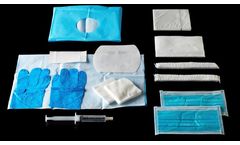
Care kits for: cannulation and line insertion, immediate or deferred infusion connection, medication reconstitution for drug delivery, filling of infusion devices, disconnection, needle withdrawing, maintaining the permeability of the vascular access, vascular access management. Hemodia designs specific drapes to fit your ...
by:XELTIS BV based inEindhoven, NETHERLANDS
The aXess graft is a restorative, synthetic, electrospun blood vessel for arteriovenous hemodialyisis access, overtime turning into a living vessel made of patient’s own ...
Manufactured by:SyncMedical LLC based inStoughton, MASSACHUSETTS (USA)
The Primo Port is a fully implantable vascular access device that meets the requirements of clinicians and patients by providing long-term repeated access to the vascular system. The Primo Port system can be used for infusion of medications, IV fluids, parenteral nutrition and blood products as well as for the aspiration of blood ...
Manufactured by:AllVascular Pty Ltd based inLeonards, AUSTRALIA
The Implant; Long term arterial implant anastomosed to either the femoral or axillary artery. Dual Dacron cuffs provide infection barrier and tissue anchorage. ePTFE vascular graft allows for end-to-side ...
Manufactured by:Simulab Corporation based inSeattle, WASHINGTON (USA)
Practice both Femoral Vascular Access as well as Femoral Regional Anesthesia with Simulab's Regional Anesthesia and Vascular Access Femoral Training Package. The package includes everything you need to build on your practice. Start your training on the Venipuncture Pad with Nerves, move to femoral access with the ...
Manufactured by:Bioteque Corporation based inTaipei, TAIWAN
Curves suitable for imaging cerebral vasculature. Soft, radiopaque nylon for minimal vessel trauma. Three-part Braided nylon designs are typically used for applications that require high torque, burst pressure resistance, pushability, steerability and kink resistance(Optimum torque control and kink resistance due to its double mesh). ...
Manufactured by:Covalon Technologies Ltd based inMississauga, ONTARIO (CANADA)
IV Clear™ Antimicrobial Clear Silicone Adhesive Securement Dressing with Chlorhexidine and Silver is used to cover and protect insertion sites, and secure devices to skin. ...
Manufactured by:Covalon Technologies Ltd based inMississauga, ONTARIO (CANADA)
CovaClear IV, the newest addition to Covalon’s vascular access portfolio, uses the same trusted silicone adhesive technology to protect patients from skin injuries and promote healing in patients who either don’t require, or cant tolerate, ...
Manufactured by:Covalon Technologies Ltd based inMississauga, ONTARIO (CANADA)
CovaView IV is a thin film dressing designed to be cover and protect IV sites. Its polyurethane backing is breathable and maintains an effective barrier against viruses, bacteria, and other external ...
Manufactured by:Cook Medical LLC based inBloomington, INDIANA (USA)
The central venous catheter is designed for treatment of critically ill patients and is suggested for: 1. Continuous or intermittent drug infusions; 2. Central venous blood pressure monitoring (CVP); 3. Acute hyperalimentation; 4. Blood sampling; 5. Delivery of whole blood or blood products; 6. Simultaneous, separate infusion of drugs. ...
Manufactured by:Healthline Medical Products based inSalt Lake City, UTAH (USA)
Peripherally Inserted Central Catheters with Power Injectability. Health Line’s single, dual, and triple lumen power PICC line kits are designed for nurses, doctors, and interventional radiologists of all experience levels. The SYNERGY CT PICC™ combines the ease and efficiency of PICC access with power injectability in multiple PICC line kit configurations to provide healthcare ...
Manufactured by:Healthline Medical Products based inSalt Lake City, UTAH (USA)
Permanent Silicone Hemodialysis Catheters for Chronic Dialysis Therapy. Health Line’s CASCADETM tunneled dialysis catheter kits come in a variety of lengths and kit configurations. Intended for long-term implantation for dialysis and apheresis therapy, these 13.5Fr hemodialysis catheters and aphaeresis catheters allow high flow rates and decrease patient therapy ...
Manufactured by:Healthline Medical Products based inSalt Lake City, UTAH (USA)
CT PICC Line MST Tray with a Maximal Barrier Tray. Designed with CDC and INS guidelines in mind, the Health Line CT PICC™ Line Max Barrier Kit combines everything clinicians require to meet their complete catheter insertion needs in one maximal barrier tray. The components in our Max Barrier Kit help healthcare providers achieve standard, safe, and easy ...
Manufactured by:Healthline Medical Products based inSalt Lake City, UTAH (USA)
Permanent Hemodialysis Catheters for Chronic Dialysis Therapy. The TAURUS™ dialysis catheter is indicated for use in attaining long-term vascular access for hemodialysis therapy. Available in 14.5Fr diameter, these tunneled polyurethane dialysis catheter kits come in a range of lengths and kit configurations that allow high flow rates and decrease patient ...
Manufactured by:Healthline Medical Products based inSalt Lake City, UTAH (USA)
The Health Line CT Midline Catheter is intended for short-term (less than 30 days) peripheral access to the venous system for the purpose of intravenous therapy, medicines, and blood products. A single midline IV access can meet infusion therapy requirements for patients needing 5 to 7 days of therapy, eliminating multiple needle sticks and enhancing patient comfort. Additionally, our midline ...
Manufactured by:Healthline Medical Products based inSalt Lake City, UTAH (USA)
The ORION™ Central Venous Catheter is designed for short-term (less than 30 days) access to the central venous system for intravenous administration. This non-tunneled CVC is available in a variety of sizes and lengths to give clinicians multiple options to meet patients’ ...
Manufactured by:Healthline Medical Products based inSalt Lake City, UTAH (USA)
The ORION™ II CT Central Venous Catheters are power injectable and intended for short-term infusion therapy. Available in a variety of sizes and lengths, the ORION™ II has an indwelling central line indicated for pressure injection of contrast media, which negates the need for another type of venous access. Health Line’s non-tunneled power injectable CVC catheter kits streamline ...
Manufactured by:Vygon SAS based inEcouen, FRANCE
Catheter in radiopaque and transparent PUR, can be used for venous or arterial access. Supplied with a small 2 way stopcock and identification plugs (red for arterial use, blue for venous use). Actual distance from the distal tip is printed numerically from 4 to 25 ...
Manufactured by:Vygon SAS based inEcouen, FRANCE
Umbilical polyurethane catheter, totally radiopaque with Expert technology, containing the AgION™ antimicrobial agent made of silver ions integrated within the wall of the catheter.The Expert catheters release silver ions from their internal and external surfaces, so the catheter offers both extraluminal and intraluminal protective action.Because the silver ions are sensitive to light ...