Refine by
Applications
Void Filling Bone Equipment & Supplies
52 equipment items found
Manufactured by:Parametrics Medical based inLeander, TEXAS (USA)
OsteoSource™ allograft bone particulates have osteoconductive properties and are well suited to fill bone structure defects. Osteoconductive allografts act as a scaffold for the growth of natural bone. Over time, the natural bone replaces the donor’s bone. OsteoSource™ eliminates the need for autograft bone, associated with donor site morbidity, longer surgery time, and ...
Manufactured by:CAM Bioceramics B.V. based inLeiden, NETHERLANDS
Porous blocks with interconnected pores that are mainly used as raw material for manufacturing bone void fillers. Macropores, with diameters of approximately 600 µm, enable cell attachment, and the open interconnected porosity enables blood circulation. This specific morphology allows faster resorption of the ceramic material and accelerates bone ...
Manufactured by:Zetagen Therapeutics Inc. based inSyracuse, NEW YORK (USA)
ZetaBase is an osteo-conductive and osteo-promotive therapy and is intended for the use as a bone void filler for bony voids or gaps that are not intrinsic to the stability of the bony structure. ZetaBase Bone Graft can be used as a bone graft ...
Manufactured by:Kinamed Incorporated based inCamarillo, CALIFORNIA (USA)
A simple set of instruments allows for precise creation of two compound wedges of bone. Transposing these wedges medializes the tubercle. ...
by:In2Bones Global based inMemphis, TENNESSEE (USA)
The i2b Resorbable Bead Kit is designed to create easy-to-use bio-resorbable beads for filling osseous defects. i2b® Resorbable Bead Kits allow surgeons to cast 100% pure synthetic resorbable bone void filler Beads in two sizes: 3.5 and 4.5mm. The beads provide a safe alternative to human or animal cadaver bone that ...
Manufactured by:Osteogenics Biomedical based inLubbock, TEXAS (USA)
Zcore™ is an osteoconductive, porous, anorganic bone mineral with a carbonate apatite structure derived from porcine cancellous bone. Proprietary processing steps preserve both interconnecting macroscopic and microscopic porous structures of Zcore™ that helps support the formation and in-growth of new ...
Manufactured by:Osteogenics Biomedical based inLubbock, TEXAS (USA)
Zcore™ is an osteoconductive, porous, anorganic bone mineral with a carbonate apatite structure derived from porcine cancellous bone. Proprietary processing steps preserve both interconnecting macroscopic and microscopic porous structures of Zcore™ that helps support the formation and in-growth of new bone. ...
Manufactured by:Cervos Medical based inMarshfield, MASSACHUSETTS (USA)
The CER-SUB-1560 is a bone access device that has a 15 gauge cannula with an open tip and is designed to be powered by a drill. The device can serve as an access port to transplant tissue or medications. For example, marrow aspirate obtained using CER-EXT can be used with the CER-SUB-1560 to transplant marrow aspirate. The mechanism your body uses to repair and maintain tissue is Vasculogenesis, ...
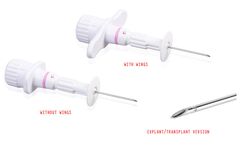
Manufactured by:Medax Srl based inSan Possidonio (MO), ITALY
Bone Marrow aspiration system “XS”, also available in the explant/transplant version ...
Manufactured by:Biedermann Motech GmbH & Co. KG based inVillingen, GERMANY
Sophisticated technology for fixation of bone fractures and bone reconstructions of the humerus. Can be utilized to deliver injectable bone void fillers to a surgical site. Compatible with MDS humerus plating systems. Innovative option for additional stability in osteoporotic and complex ...
Manufactured by:Leader Biomedical Group based inAmsterdam, NETHERLANDS
Cancellous and cortical bone chips with sound structural and mechanical properties for filling of bone loss and/or correcting of ...
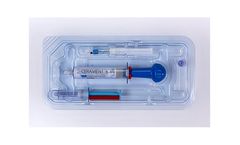
Manufactured by:BONESUPPORT AB based inLund, SWEDEN
CERAMENT V is an injectable synthetic bone void filler consisting of 40% hydroxyapatite, 60% calcium sulfate and the antibiotic vancomycin ...
Manufactured by:Spineology Inc. based inSt. Paul, MINNESOTA (USA)
Incite™ Cortical Fibers are a unique and versatile bone grafting option. A proprietary processing method provides thin bone fibers which expose significant osteoconductive surface area and osteoinductve endogenous growth factors. The ultrafine, entangled fibers are highly malleable to provide exceptional handling properties and support a variety of delivery options for maximum operative ...
Manufactured by:BONESUPPORT AB based inLund, SWEDEN
CERAMENT BONE VOID FILLER is an injectable, moldable and drillable synthetic bone void filler consisting of 40% hydroxyapatite, 60% calcium sulfate and the radio-contrast agent ...
Manufactured by:OrthoPediatrics Corp. based inWarsaw, INDIANA (USA)
CERAMENT BONE VOID FILLER is an injectable, moldable and drillable synthetic bone void filler consisting of 40% hydroxyapatite, 60% calcium sulfate and the radio-contrast agent iohexol. The unique ratio of hydroxyapatite and calcium sulfate is designed to enable CERAMENT to resorb at the same rate that bone forms. Calcium sulfate acts as a resorbable carrier for hydroxyapatite, and hydroxyapatite ...
Manufactured by:Surgentec LLC based inBoca Raton, FLORIDA (USA)
Surgentec brings a new advancement to the bone graft delivery arena with the Graftgun. Our Graft Delivery System (GDS®) is designed to allow for universal, quick, and accurate bone graft delivery to a surgical site without the problems of a traditional funnel.Its patented, controlled delivery method is designed to safely dispense bone graft ...
Manufactured by:SpineVision based inAntony, FRANCE
Smart’OS is a bone substitute combining in both granules or putty a micro & macroporous structure with the well known association of β-TCP & Hydroxy Apatite to enhance bone fusion. ...
by:Geistlich Pharma North America Inc. based inPrinceton, NEW YORK (USA)
User Benefits: Geistlich Bio-Gide® Compressed is the twin to Geistlich Bio-Gide®, a collagen membrane for reliable bone regeneration and optimal tissue integration.1-3 In comparison, Geistlich Bio-Gide® Compressed has a smoother surface, is firmer in touch and easier to cut.4 Therefore it is especially suitable for dentists who would like alternative handling properties.4 Geistlich ...
by:Molecular Matrix, Inc. based inWest Sacramento, CALIFORNIA (USA)
Osteo-P™ Bone Graft Substitute (BGS) is a non-mineralized, synthetic bone void filler consisting of a hyper-crosslinked carbohydrate polymer. It is highly porous, biocompatible, biodegradable, and osteoconductive. Osteo-P™ BGS is intended for the filling of bone defects created surgically or as a ...
Manufactured by:BONESUPPORT AB based inLund, SWEDEN
CERAMENT G is an injectable synthetic bone void filler consisting of 40% hydroxyapatite, 60% calcium sulfate and the antibiotic gentamicin ...