

- Home
- Companies
- Aptevo Therapeutics
- Products
- ADAPTIR - Modular and Flexible ...
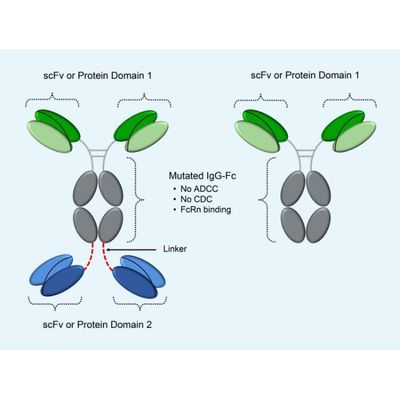
ADAPTIR - Modular and Flexible Technology
Demonstrated ability to produce monospecific and bispecific drug candidates; Leverages IgG1 Fc to form homodimeric bispecific molecules; IgG1-derived Fc can be mutated to ablate effector functions antibody-dependent cellcytotoxicity (ADCC) and complementdependent cytotoxicity (CDC), while maintaining original stability and half-life (confirmed by cell binding, cell function, SPRbased measurements of affinity, melting temperature) and good pharmaco-kinetic properties in preclinical studies; Binding domains are engineered from IgG variable regions, fragment libraries, receptors or ligands; Optimized to minimize proteolytic cleavage and post-translational modifications.
Antibody-like Half-Life and Production Processes
- Demonstrated half-life up to 12.5 days in rodents
- Standard manufacturing process with high yields and purity, demonstrated with multiple clinical candidates
- Redirected T-Cell Cytotoxicity (RTCC)
- Co-stimulation of immune receptors to activate immune responses
- Targeted cytokine delivery
Current Pipeline Candidates using ADAPTIR Technology:
- APVO436 (CD123 x CD3): Clinical candidate for the treatment of AML, MDS
- ALG.APV-527 (41BB x 5T4): Preclinical candidate, planned to advance into clinic for the treatment of solid tumors expressing 5T4
- APVO603 (41BB x OX40): Preclinical candidate that actives two costimulatory receptors on immune cells for the treatment of multiple solid tumor indications
