Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Pennsylvania
- South Carolina
- South Dakota
- Texas
- Utah
- Virginia
- Washington
- Wisconsin
Applications
- Prefilled Syringes and Combination Products for Nitrogen Dioxide Gas Sterilization
- Personal ECG device solutions for biopharma sector
- Klarity - Built Therapeutic Platform Technologies for Gastrointestinal Disease
- Klarity - Built Therapeutic Platform Technologies for Neurological Disorders
- Klarity - Built Therapeutic Platform Technologies for Metabolism
Biopharma To Participate In Panel Discussion On Investment Opportunities In China Hosted By Duane Morris And The Chinese Biopharmaceutical Equipment Supplied In Usa
115 equipment items found
Premium
Manufactured by:Environmental Specialties, LLC an EMCOR Group, Inc. based inRaleigh, NORTH CAROLINA (USA)
Our popular Low Temp Walk-In Suite (LTW) utilizes an innovative chamber-within-a-chamber design to support the stringent biorepository and pharmaceutical requirements for ultra-cold temperature storage freezer vaults. It offers a more energy ef?cient alternative to the traditional “freezer farm” approach, while still delivering maximum capacity, multi-temperature storage ?exibility, ...
Premium
Manufactured by:AB Sciex LLC based inFramingham, MASSACHUSETTS (USA)
Perform consistent and compliant biotherapeutics characterization faster than ever. With the PA 800 Plus system, you can confidently safeguard the success of your biologics. Run multiple characterizations from a single system that biopharma labs depend on, and produce qualitative and quantitative analyses, with speed and ...
Manufactured by:Actylis based inPort Washington, NEW YORK (USA)
3-Hydroxy-2,7-Naphthalenedisulfonic Acid Disodium ...
Manufactured by:Actylis based inPort Washington, NEW YORK (USA)
18000197 / (+)-10-Camphorsulfonic Acid. Empirical Formula: C10H16O4S. CAS Number: 3144-16-9. Molecular Weight: 232.3 g/mol. EC Number: ...
Manufactured by:S1 Biopharma, Inc. based inNew York City, NEW YORK (USA)
Orexa is S1 Biopharma’s first therapy for erectile dysfunction ...
Manufactured by:S1 Biopharma, Inc. based inNew York City, NEW YORK (USA)
S1B-307 is at the IND / Phase 1 preparatory stage for the treatment of orgasmic disorder (OD). S1 Biopharma expects 505(b)(2) status to be granted for this ...
Manufactured by:Vante Biopharm - Sebra, Brand of Machine Solutions Inc. based inTucson, ARIZONA (USA)
The TPE Sealer produces consistent, quality seals on most TPE tubing used in biopharmaceutical manufacturing. This sealer is easy to clean, portable and compact. One-button operation makes this sealer easy to use and validate. The unique remote thin wall sealing head clamps and hangs from tubing for convenient use with manifold systems. The Thin Wall Sealing Head seals thin wall (.062) tubing: ...
Manufactured by:Aquasyn LLC based inCarson City, NEVADA (USA)
Aquasyn’s diaphragms are unlike any others in the biopharmaceutical industry. Our diaphragms are precision molded to tight tolerances for superior service life and crafted of approved materials which will not sacrifice the purity of the most sensitive of ...
Manufactured by:Vitro Biopharma based inGolden, COLORADO (USA)
High passage capabilities. Expands in VitroPlusIII Low Serum, Complete Medium (Vitro Biopharma, Cat. No. PC00B1–optimized for high growth rates, reduced doubling times, healthy cells, and stability). Able to grow in low oxygen (1% - 5% O2). Available for research ...
Manufactured by:Vitro Biopharma based inGolden, COLORADO (USA)
Human Pancreatic Fibroblasts. Cryopreserved at a low passage. High passage capabilities. Expands in VitroPlusIII Low Serum, Complete Medium (Vitro Biopharma, Cat. No. PC00B1-optimized for high growth rates, reduced doubling times, healthy cells, and stability). Able to grow in low oxygen (1% - 5% O2). Available for research ...
Manufactured by:Vitro Biopharma based inGolden, COLORADO (USA)
Human Ovarian Serous Cancer Associated Fibroblasts. Cryopreserved at a low passage. High passage capabilities. Expands in VitroPlusIII Low Serum, Complete Medium (Vitro Biopharma, Cat. No. PC00B1–optimized for high growth rates, reduced doubling times, healthy cells, and stability). Able to grow in low oxygen (1% - 5% O2). Available for research ...
Manufactured by:Vitro Biopharma based inGolden, COLORADO (USA)
Human Mesenchymal Stem Cells are isolated from the umbilical cord; Cryopreserved at a low passage; High passage—able to pass 5-10 passages; Expands in Low Serum, Complete Medium (Vitro Biopharma, Cat. No. SC00B1—optimized for high growth rates, reduced doubling times, healthy cells and stability); Able to grow in low oxygen (1% - 5% O2); Available for research ...
Manufactured by:Vitro Biopharma based inGolden, COLORADO (USA)
Human Mesenchymal Stem Cells are isolated from placenta; Cryopreserved at a low passage; High passage–able to pass 5-8 passages; Expands in Low Serum, Complete Medium (Vitro Biopharma Cat. No. SC00B1–optimized for high growth rates, reduced doubling times, healthy cells, and stability); Able to grow in low oxygen (1% - 5% O2); Available for research ...
Manufactured by:Altucell, Inc. based inNew York, NEW YORK (USA)
Encapsulated cell therapy is an emerging area of biopharmaceutical research that aims to unleash the therapeutic potential of cells to treat serious diseases without the need for immunosuppression. Altucell has developed patented, proprietary engineered cells that are combined with a patented encapsulation technology to create effective ...
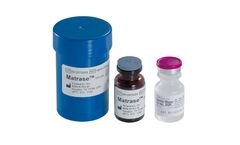
Manufactured by:InGeneron, Inc. based inHouston, TEXAS (USA)
InGeneron’s proprietary MatraseTM Reagent is a high purity enzymatic mixture for the release of mesenchymal stem cells and other regenerative cells from adipose tissue. The product is manufactured under a contract by a major biopharmaceutical company under current Good Manufacturing Practices (cGMP) and tested by high-performance liquid chromatography. MatraseTM Reagent is free of mammalian ...
Manufactured by:Scarab Genomics, LLC based inMadison, WISCONSIN (USA)
Our patented C-Flow™ continuous fermentation process is designed to produce large quantities of biopharmaceuticals with small scale inexpensive equipment, saving dramatically on costs. The C-Flow™ process was developed using Clean Genome® E. coli strains expressing recombinant CRM197, a carrier protein used to enhance the immunogenicity of poorly immunogenic carbohydrates in ...
by:Sorrento Therapeutics, Inc. based inSan Diego, CALIFORNIA (USA)
Sorrento’s proprietary G-MAB technology, invented by Dr. Ji, is based on the use of RNA transcription for amplification of the antibody variable domains from over 600 donors. In-depth analysis of deep sequencing DNA data showed that the G-MAB library contains more than 10 quadrillion (1016) distinct antibody sequences. This makes it one of the largest fully human antibody libraries in the ...
Manufactured by:Creative Biogene - Microbiotec based inShirley, NEW YORK (USA)
Microbial enzymes have been used in a large number of fields, such as chemical, agricultural and biopharmaceutical industries. Our enzyme production services are based on bacteria, fungi, and yeast, from strain selection, optimization, and process development to scale-up ...
Manufactured by:Vitro Biopharma based inGolden, COLORADO (USA)
Human Lung Adenocarcinoma Cancer Associated Fibroblasts; Cryopreserved at a low passage; Stage: III; High passage capabilities; Expands in VitroPlusIII Low Serum, Complete Medium (Vitro Biopharma, Cat. No. PC00B1–optimized for high growth rates, reduced doubling times, healthy cells, and stability); Able to grow in low oxygen (1% - 5% O2); Available for research ...
Manufactured by:BioFactura, Inc. based inFrederick, MARYLAND (USA)
BioFactura develops and commercializes high-value biosimilars (i.e., follow-on biologics or generic biopharmaceuticals) using its patented StableFastTM Biomanufacturing Platform, the optimal choice for bringing these drug to market with faster, lower cost, superior-quality manufacture. The global biosimilars market is projected to grow from $4.5B in 2017 to over $23B by 2023 with a Compound ...