Refine by
Bone Marrow Cell Equipment Supplied In Usa
50 equipment items found
Manufactured by:Biocardia, Inc. based inSunnyvale, CALIFORNIA (USA)
The investigational CardiAMP™ Cell Therapy is designed to be a comprehensive biotherapeutic heart failure solution, incorporating: a proprietary molecular diagnostic to characterize the potency of a patient’s own bone marrow cells and determine if they are an optimal candidate for therapy. a point of care processing ...
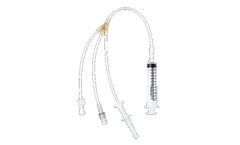
Manufactured by:Charter Medical, LLC based inWinston-Salem, NORTH CAROLINA (USA)
Charter Medical’s Advect® Fluid Transfer Sets are designed to support the quality and manufacturing requirements of personalized care. The transfer sets are intended for sterile transfer of bone marrow mononuclear cells from one container to another. ...
Manufactured by:LifeTein, LLC based inHillsborough, NEW JERSEY (USA)
Macrophage Colony Stimulating Factor (M-CSF), also known as CSF-1, can be produced by a number of cells, including fibroblasts, monocytes, activated macrophages, secretory epithelial cells of the endometrium, endothelial cells activated by LPS or cytokines, and bone marrow stromal cells. In ...
Distributed by:Crescent Chemical Company based inIslandia, NEW YORK (USA)
Crescent Chemical Co., Inc. can supply Ultroser G Serum Substitute. Ultroser Gis manufactured by BioSepra in France. Importation of Ultroser G requires a USDA Importation Permit. Crescent Chemical is happy to help you apply for this ...
Manufactured by:Alliance Spine based inSan Antonio, TEXAS (USA)
Bone Marrow Concentrate System utilizes a proprietary system of gentle centrifugation and protection of the delicate buffycoat layer to produce superior cell yields for Bone Marrow Concentrate ...
Manufactured by:Trailhead Biosystems Inc. based inBeachwood, OHIO (USA)
We are developing human long-term hematopoietic stem cells (LT-HSCs) from human (non-embryo) induced pluripotent stem cells (iPSCs). HSCs are rare subsets of hematopoietic cells that are responsible for the life-long production of all blood cell lineages, and for the reconstitution of bone marrow ...
by:Precipio, Inc based inNew Haven, CONNECTICUT (USA)
IV-Cell universal culture media is a Research-use only (RUO), ready-to-use, fully supplemented medium developed specifically to support bone marrow and peripheral blood cell culture for in vitro cytogenetic analysis of hematological disease. IV-Cell is a proprietary culture media that does not require the ...
Manufactured by:Creative BioMart based in, NEW YORK (USA)
IL-4 is a pleiotropic cytokine that regulates diverse T and B cell responses including cell proliferation, survival and gene expression. Produced by mast cells, T cells and bone marrow stromal cells, IL-4 regulates the differentiation of naive CD4+ T cells into ...
Manufactured by:Biocardia, Inc. based inSunnyvale, CALIFORNIA (USA)
Where the CardiAMP Cell Therapy System uses a patient’s own cells, the CardiALLO system uses culture-expanded, bone marrow-derived mesenchymal stem cells from a universal donor. This allows the cells to be obtained "off the shelf" from a pharmacy, negating the need for a ...
Manufactured by:Rocket Pharmaceuticals, Inc. based inCranbury, NEW JERSEY (USA)
FA is a rare, genetic disorder affecting DNA repair. Approximately two-thirds of FA cases are caused by genetic defects in the FANCA gene, which results in the FA subtype known as FA Complementation Group A (FA-A). FA patients may develop bone marrow failure (very low blood counts), cancers of the blood or other ...
Manufactured by:Taconic Biosciences, Inc. based inRensselaer, NEW YORK (USA)
Origin: Taconic received the NCr nude Spontaneous mutant model from NCI in 1993 after several years of random breeding. The mice were derived by hysterectomy to achieve germ-free status prior to introduction into Restricted Flora™ IBU™ colonies. These mice have both BALB/c inbred and NIH(S) outbred stock in their genetic background. ...
by:Gain Therapeutics, Inc. based inBethesda, MARYLAND (USA)
Very limited and investigational symptomatic treatment options include substrate reduction therapy, enzyme replacement therapy, bone marrow transplantation, stem cell transplantation and gene therapy to address non-CNS symptoms. Gain is developing allosteric regulators that are designed to decrease toxic substrate accumulation in organs and ...
Manufactured by:Precision For Medicine, Inc. based inFrederick, MARYLAND (USA)
Advancing your research into hematological malignancies, immunology, and other related areas requires access to high quality human bone marrow mononuclear cells. Precision for Medicine has an extensive inventory of normal, healthy donors with longitudinal data and ability to recall for additional biospecimen ...
Manufactured by:ADS Biotec Inc. based inOmaha, NEBRASKA (USA)
The HANABI-PII Plus reduces hands-on time of the entire harvesting process by 75%, allowing time for other specialized tasks and providing increased throughput for your laboratory. Capable of processing up to 24 samples per run and it eliminates variability compared to manual processing methods. The HANABI-PII Plus can be integrated with additional options such as reagent sensors, waste fluid ...
by:Gain Therapeutics, Inc. based inBethesda, MARYLAND (USA)
Very limited and investigational symptomatic treatment options include substrate reduction therapy, enzyme replacement therapy, bone marrow transplantation, stem cell transplantation and gene therapy to address non-CNS symptoms. Gain is developing allosteric regulators that are designed to decrease toxic substrate accumulation in organs and ...
Manufactured by:StemBioSys, Inc. based inSan Antonio, TEXAS (USA)
CELLvo™ Matrix is the only commercially available substrate that aims to recapitulate biochemical as well as structural and mechanical cues of the native niche by leaving the extracellular matrix intact. Cell-derived matrices create a biologically-relevant culture environment for a variety of cell types by providing all the cues cells expect to receive in vivo in our culture dishes. This ...
Manufactured by:ADS Biotec Inc. based inOmaha, NEBRASKA (USA)
The HANABI-PI is specifically created for small to mid-size throughput, enabling processing of up to 16 suspension cultures per run and eliminating variability compared to manual processing methods. Walk away reliability and low maintenance reduces FTE time associated with the manual harvesting process up to 75%. The HANABI-PI can be integrated with additional options such as reagent sensors, ...
Manufactured by:MYCO Medical based inApex, NORTH CAROLINA (USA)
RELI BEN Bone Marrow Biopsy Needle, Trocar point, 11G x 10cm, ...
Manufactured by:ADS Biotec Inc. based inOmaha, NEBRASKA (USA)
The HANABI-PIII Plus reduces hands-on time of the entire harvesting process by 75%, allowing time for other specialized tasks, providing increased throughput for your laboratory. Specifically created for large-size samples throughput, the HANABI-PIII Plus enables processing up to 64 samples per run and eliminates variability compared to manual processing methods. The HANABI-PIII Plus can be ...
by:Aegle Therapeutics based inWoburn, MASSACHUSETTS (USA)
Epidermolysis bullosa (“EB”) is a group of rare genetic disorders that manifests as blistering or erosion of the skin in response to little or no apparent trauma. There are many genetic and symptomatic variations of EB. EB is always painful, often pervasive and debilitating, and is in some cases lethal before the age of 30. EB affects both genders and every racial and ethnic ...